Askari et al (2012)
 The following study can be used to support the use of drug treatments for people with OCD. It focuses on granisetron, a drug that reduces serotonergic activity by binding to 5-HT(3) receptors on the postsynaptic membrane.
The following study can be used to support the use of drug treatments for people with OCD. It focuses on granisetron, a drug that reduces serotonergic activity by binding to 5-HT(3) receptors on the postsynaptic membrane.
This study may also be used on Paper 1 to answer a Biological Approach short answer questions about neurotransmission, including questions focused specifically on antagonists.
Initially, patients with OCD were treated with tricyclic antidepressants such as clomipramine, but this drug presented a range of problems including side effects, tolerance, and withdrawal symptoms. SSRIs and SNRIs soon became popular alternatives, but more recently researchers have explored the use of serotonin 5-HT3 receptor antagonists. An antagonist is a medication that typically binds to a receptor without activating them, but instead, decreases the receptors' ability to be activated by another agonist.
One such drug had already proved effective (ondansetron) but the researchers wished to focus on another drug, granisetron, which they hoped would be tolerated better by patients, have reduced interactions with other drugs, and could give longer-lasting results.
The study below is a good example of how researchers test for the effectiveness of a drug treatment.
The aim of this study was to examine the efficacy and tolerability of granisetron when combined with an SSRI called fluvoxamine, for patients with moderate to severe OCD.
The sample was recruited from outpatient clinics at two large referral centers in Iran. 39 participants aged 18 to 60 were included in the final analysis. All had Y-BOCS scores of at least 21/40, (moderate symptoms) and reached the threshold for a diagnosis of OCD, using the DSM-IV-TR.
Participants were randomly allocated to either the experimental or the control group. Each received 1 mg of either granisetron or the placebo every 12 hours, in addition to 100mg of fluvoxamine for the first four weeks, increasing to 200mg in the remaining weeks.
Obsessions and compulsions were assessed using the sub-scales of the Y-BOCs and side-effects were monitored using a standardized checklist. Data were collected at the start of the study, (the baseline), and every other week, up to and including week 8. Neither the participants nor the researchers were aware of which group the participants were in, i.e. it was a double-blind study.
The dependent variable was the extent to which symptoms improved over the course of the 8 weeks and this was divided into three categories.
Partial response: a reduction in symptoms of least 25% on the Y-BOCs
Complete response: a reduction of at least 35% on the Y-BOCs
Remission: a Y-BOCS score equal to or less than 16
Over the course of the 8-week trial, the granisetron group experienced a reduction in symptoms equivalent to 16.8 points on the Y-BOCs, whereas the placebo group only reduced their scores by an average of 9.9 points. 100% of the granisetron group achieved a complete response at 8 weeks and 90% were classed as in remission compared with only 35% of the placebo (+ SSRI) group. Tolerability was the same for both groups, suggesting the side effects of granisetron were no worse than the SSRI on its own.
At two weeks, the difference between the granisetron and placebo groups was not significant, but by week four the granisetron group was showing significantly greater improvement than the placebo group, as illustrated in the table and graph below.
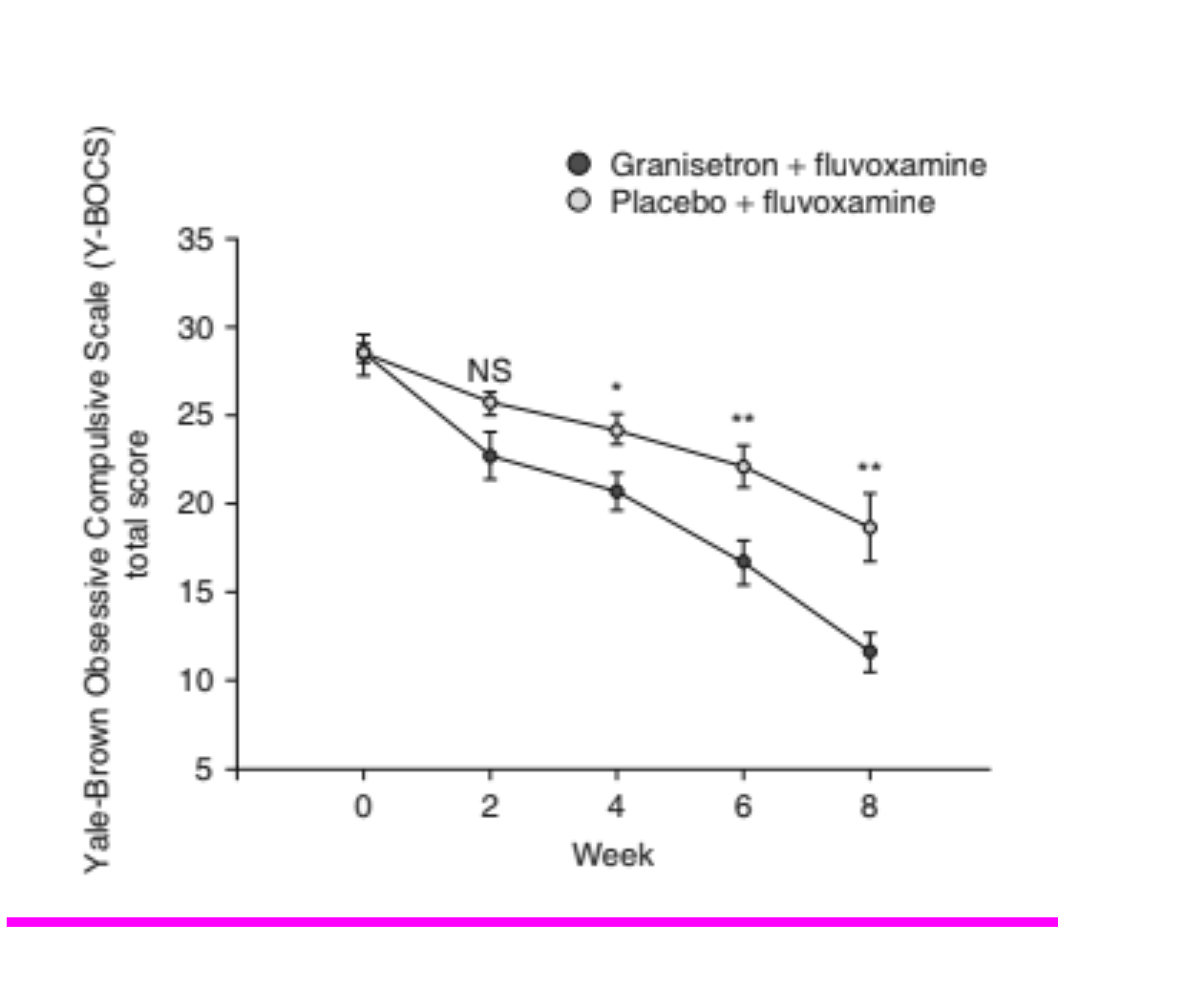 The line graph shows the reduction in Y-BOCS scores over the eight-week period, and clearly shows the superiority of the granisetron group compared with the placebo group. The graph also shows how the difference between the two groups failed to reach significance at week 2, although it was in the expected direction. However, the difference was significant at week 4 (p < 0.05), and was highly significant at weeks 6 and 8, (p < 0.01).
The line graph shows the reduction in Y-BOCS scores over the eight-week period, and clearly shows the superiority of the granisetron group compared with the placebo group. The graph also shows how the difference between the two groups failed to reach significance at week 2, although it was in the expected direction. However, the difference was significant at week 4 (p < 0.05), and was highly significant at weeks 6 and 8, (p < 0.01).
The researchers concluded that people with OCD can achieve a faster and greater reduction in symptoms through combining fluvoxamine and granisetron, with no increase in adverse side effects.
The internal validity of the study was high as the study used random allocation to ensure the reduction in symptoms could not be attributable to participant variables;
The validity of the participants’ OCD diagnosis was carefully checked by two experienced psychiatrists using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I);
This is a pioneering study and at the time of publication it had not been replicated, meaning the reliability of the findings had not been established;
The study only lasted eight weeks meaning the longevity of these initial findings is unclear; tolerance may have appeared had the patients been monitored over a longer period, eg. 12 to 18 months;
The study included slightly more females than males, and the mean age was mid-thirties, meaning males and patients who were younger or older may have behaved differently. In addition, the study did not include anyone who had already been classed as treatment-resistant meaning it is unclear whether granisetron would be helpful for everyone;
Inter-rater reliability between the four residents responsible for assessing the patients was high ( > 85%) meaning they were generally in agreement with each other’s scoring of the Y-BOCs;
The participants, psychiatrists, residents (in charge of prescribing medications and assessing symptoms), and the statistician who analyzed the data were all ‘blind’, i.e. did not know which groups the participants were in meaning the results could not be affected by demand characteristics or researcher bias.

 IB Docs (2) Team
IB Docs (2) Team
