Biological explanations of obesity
 Biologists have identified several hormones that play a key role in eating and the way our body uses energy and stores fat. These hormones include cortisol, insulin, leptin, and ghrelin. In spite of research that shows that these hormones let us know when we are hungry and when we have had enough food, there is still doubt as to their role in obesity.
Biologists have identified several hormones that play a key role in eating and the way our body uses energy and stores fat. These hormones include cortisol, insulin, leptin, and ghrelin. In spite of research that shows that these hormones let us know when we are hungry and when we have had enough food, there is still doubt as to their role in obesity.
Another theory is that obesity may be the result of a genetic predisposition. It seems that body size runs in families. Having one obese parent results in a 40 percent chance of becoming obese, and having two obese parents increases the likelihood of becoming obese to 80 percent.
The role of hormones
One biological theory of over-eating and obesity is that there is an imbalance of hormones. As you will see in the chart below, there are several hormones that play a role in our eating behaviour. Being able to isolate a single hormone to argue that it plays a key role in obesity has not yet been possible. It is highly likely that all of these hormones may play a key role and even more likely that there is some level of interaction of the hormones.
Hormones that play a role in eating and fat storage
High levels of cortisol can, for example, increase appetite with a preference for “comfort food” and cause white adipose (fat) tissue to redistribute to the abdominal region, which may ultimately lead to abdominal obesity.
Ghrelin is secreted by the stomach and is often called the "hunger hormone." Ghrelin tells the hypothalamus that you are hungry and that you need to eat. High levels of ghrelin are associated with over-eating and obesity.
Insulin is a hormone secreted by the pancreas that allows your body to use glucose from carbohydrates in the food that you eat for energy or to store glucose for future use. Insulin resistance is when the body does not respond appropriately to insulin, leading to type II diabetes. There is a correlation between insulin resistance and obesity.
Leptin is secreted by adipose tissue (fat cells) and tells the hypothalamus that we have had enough to eat. Leptin resistance is thought to play a role in obesity.
Ghrelin tells us that we need to eat. When glucose levels in the blood fall, ghrelin levels rise, telling the brain that we need to eat to get more energy. Could this hormone play a role in obesity?
Interestingly, ghrelin levels are lower in individuals with a higher body weight compared with lean individuals, which suggests ghrelin could be involved in the long-term regulation of body weight. One would expect higher levels in people with obesity. However, ghrelin levels are usually lower in people with higher body weight compared with lean people, which suggests ghrelin is not a cause of obesity, but may influence eating behaviours that may contribute to obesity.
Goldstone (2010) carried out a study with 22 nonobese adults (17 men and 5 women). The design was a repeated measures design where the participants either skipped breakfast or came to the lab 90 minutes after eating breakfast. In the case where participants came to the lab after having eaten breakfast, on one visit they were injected with a saline solution and on another visit they were injected with ghrelin. The experiment was a double-blind control where neither the researcher nor the participants knew which injection was given.
In each condition, the participants were shown pictures of high-calorie foods such as chocolate cake and pizza. They were also shown low-calorie foods such as salads, vegetables, and fish. Using a keypad, the participants were asked to rate how appealing they found each food picture. Overall, the high-calorie foods were more appealing in all conditions. However, high-calorie foods, especially sweet foods, were of greater appeal when the participants fasted and when they received ghrelin after eating breakfast. But we know that ghrelin makes us hungry, so why does this matter? What may matter is both why ghrelin levels may increase and what we choose to eat when our ghrelin levels of high.
Research has shown that cortisol may play a key role in the increase in ghrelin (for example, Azzam et al, 2017). This may mean that stress and sleep deprivation - both of which raise one's level of cortisol - may play a role in increased ghrelin levels (and decreased leptin levels) and therefore unhealthy eating patterns. Brondel et al (2010) carried out a study to see what the short-term effects of sleep deprivation would be on men's eating behaviour. The participants were 12 men with an average age of 22 years old and an average BMI of 22.30. The design was a repeated measures design where there were 48 hours between conditions.
In one condition, the men slept for eight hours - from midnight to 8 am - or for four hours - from 2 am - 6 am. Their eating behaviour was then observed over the next day. They were given jam on buttered toast for breakfast, a buffet for lunch, and a menu for dinner from which they could order whatever they wanted. In addition, their physical activity was measured by using an actimeter.
The researchers found that when the men slept only four hours, they consumed on average 22% more calories on the following day than when they had eight hours of sleep. They also found that physical activity was slightly higher for those with sleep deprivation as well. The researchers concluded that poor sleep hygiene over time could lead to a higher calorie intake than energy expenditure and could be a factor in obesity.
ATL: Ethics
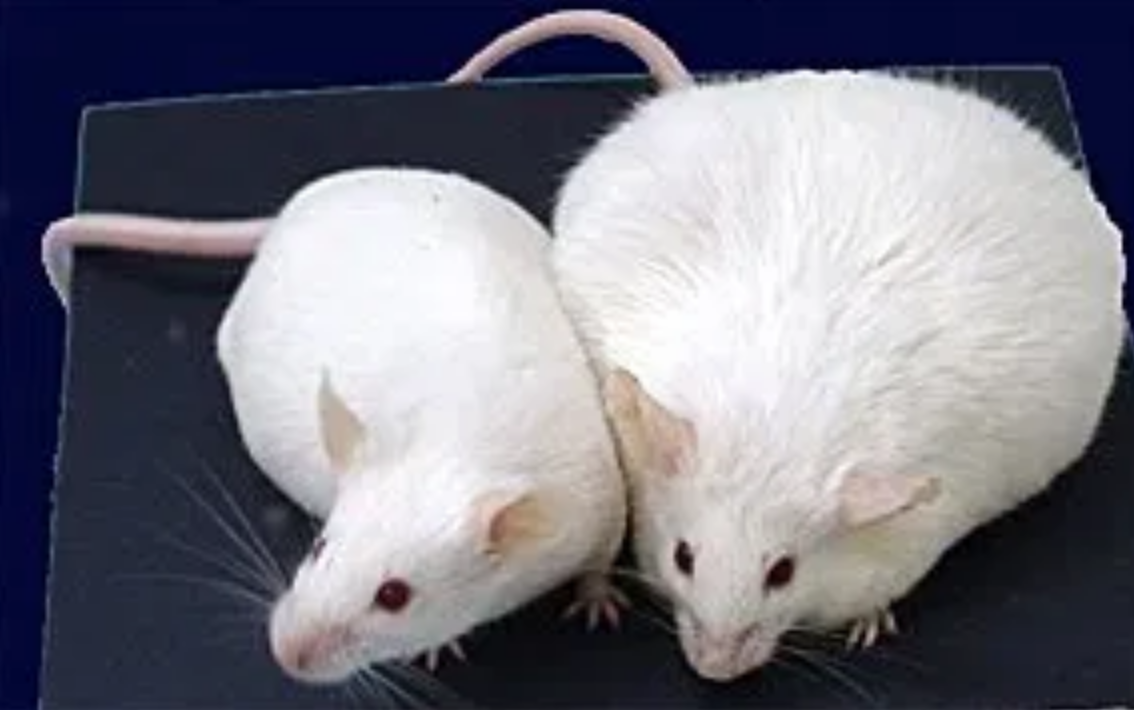 Jeffrey Friedman and his team at the Jackson Laboratory began the search for support for the lipostatic theory - that is, that there is some signal from the fat cells which give feedback to the brain in order to regulate food intake and body weight. The question was, what is that feedback mechanism?
Jeffrey Friedman and his team at the Jackson Laboratory began the search for support for the lipostatic theory - that is, that there is some signal from the fat cells which give feedback to the brain in order to regulate food intake and body weight. The question was, what is that feedback mechanism?
The team published their findings in Zhang et al (1994) in which they announced the discovery of the hormone known as leptin. They were able to find the hormone by studying the OB mouse, a mouse with a genetic mutation that leads to hyperphagia - a lack of control over eating that leads to overconsumption and obesity.
In order to carry out the study, the researchers surgically attached an OB mouse with a lean mouse so that the two animals shared their blood supply. The hypothesis was that if there was in fact a hormone that regulates weight, then it would be transferred from the lean mouse to the OB mouse. If there was, in fact, a hormone that was leading to overeating in the OB mouse, then the lean mouse would gain weight. The researchers found that by connecting to the two mice, the OB mouse did, in fact, lose weight. This put the researchers on the path to discovering leptin. 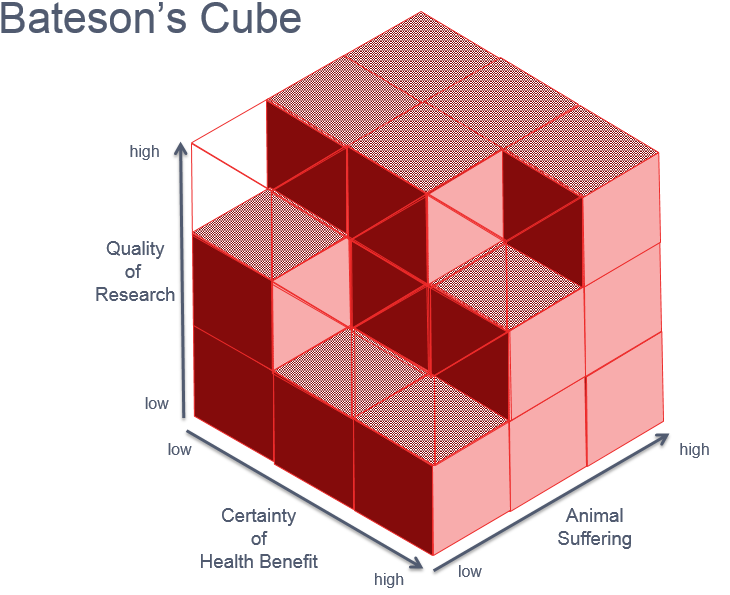
But what about the ethics of this study? Bateson's cube is a way of determining whether a study using animals was ethical or not. How would you answer the following questions?
- How well designed was the research carried out by the team?
- To what extent did the animal suffer?
- What was the certainty of the benefit of this research to animal or human health?
Bateson's cube is a way to think about the ethics of animal research. When answering the questions above, if the result is an empty cube in the model, then the research is considered to be ethical. What would you say about this study?
Leptin is the hormone that tells us that we are done eating. It gives us the feeling of being full - what biologists refer to as satiety. As our fat cells decrease because we have used up energy, our leptin levels fall (and ghrelin levels rise). When our leptin levels fall, it gives us a feeling of hunger and tells us that it is time to eat. As we eat, our body then will begin to store some of the energy as fat. As the fat cells increase, the leptin levels rise and signal to the hypothalamus that we feel full (or satiated). At this point, we then stop eating and the cycle begins all over again. So, if this is a normal process, how might it play a role in obesity?
Biologists argue that leptin resistance may play a role in obesity. Leptin resistance is the failure of leptin to signal to the hypothalamus that feeling of satiety, indicating that eating should stop. It is still not clear what exactly leads to leptin resistance; it is believed that there may be many different reasons why leptin resistance may occur. Knight et al (2010) found that when mice were given a high fat vs low-fat diet from the age of 4 weeks old, those on the high-fat diet developed leptin resistance and became obese. The difficulty is determining whether this is true also in humans. Does leptin resistance lead to obesity? Or does obesity potentially lead to leptin resistance?
ATL: Critical thinking
 Amgen, one of the world's largest independent biotechnology companies, paid 20 million USD for the commercial rights to leptin to produce a treatment for obesity. In one trial, 73 obese volunteers were injected with either a dose of leptin or a placebo. The study used a double-blind strategy.
Amgen, one of the world's largest independent biotechnology companies, paid 20 million USD for the commercial rights to leptin to produce a treatment for obesity. In one trial, 73 obese volunteers were injected with either a dose of leptin or a placebo. The study used a double-blind strategy.
The leptin supplements had some side effects, including headaches, dizziness, nausea, and abdominal pain. As a result, 47 participants did not complete the study. An example of participant attrition.
However, for those that did complete the study, there appeared to be good news. Those participants taking leptin supplements lost an average of 7.1 kg, whereas the placebo group lost only an average of 1.3 kg.
Based on your understanding of statistics, do you think that they should hurry to approve the drug?
Do a quick look online at all of the possible leptin supplements that you could buy. What would you advise a friend who thinks that this may be the solution they have been looking for?
You can find the answer in the revision presentation on biology and obesity.
Based on your understanding of statistics, do you think that they should hurry to approve the drug?
As can be seen in the presentation, the problem with these results is that they rely on the mean of the weight lost. In the high-dose group, the range of results was from a loss of 15 g to a gain of 5 kg. In addition, body mass that was lost was regained quickly after the study.
Do a quick look online at all of the possible leptin supplements that you could buy. What would you advise a friend who thinks that this may be the solution they have been looking for?
Most leptin supplements don’t actually contain the hormone. While numerous supplements are labeled as “leptin pills,” most contain a mix of various nutrients marketed to reduce inflammation and, therefore, increase leptin sensitivity.
The research on the role of leptin is rather mixed. In addition, there are clearly other ways that we can lose weight that are more natural - e.g. exercise, controlling our sugar intake. The goal of this discussion or writing task is to get students to think about the use of such supplements and to build a coherent argument for or against their use.
Genetic explanations
In order to investigate the relative role of genes and the environment, researchers can carry out twin studies. Stunkard et al (1990) studied 93 pairs of MZ twins who were raised apart. He found a concordance rate of over 65% for BMI. This means that there may be a genetic predisposition to obesity that is expressed through environmental stimuli. Although results from twin studies indicate a genetic factor in obesity, the role of this factor is not really clear. One suggestion relates to metabolism, which may be genetically determined, but the evidence is still inconclusive.
Sorensen et al (1998) carried out a study of Danish children who had been adopted. The study was a longitudinal study, following the children over a period of 23 years. The researchers found a greater correlation between the weight of the adoptees and the birth parents than between the adoptees and the adoptive parents. This seems to indicate a genetic link to obesity. However, the influence of the mother's eating habits and the early environment after birth and before the adoption took place cannot be ruled out.
Linkage analysis studies have found a link between a mutation of a gene encoding POMC (proopiomelanocortin) and obesity. POMC is associated with appetite regulation. A study by Farooqi and O'Rahilly (2006) was carried out with mice. They found that mice with a mutation of the POMC gene showed higher levels of appetite and obesity. However, this was only true when they were given a high-fat diet. When they were given a balanced diet, their weight was within the range of normal. This could be evidence of gene-environment interaction - that is, that the presence of the genetic mutation alone is not enough to cause obesity; it must be expressed by a certain type of diet. As promising as this research may appear, only about 5% of people with obesity have this genetic mutation (Bouchard, 2009).
There is evidence that genes may determine individual susceptibility to weight gain. However, the obesity epidemic cannot be attributable to genetic factors alone, since the increase in the prevalence of obesity has taken place over too short a period for the genetic make-up of the population to have changed substantially. Changes in culture and food availability - together with this genetic predisposition - may explain the steep increase in obesity worldwide.
Research in psychology: The Dutch Hunger Winter Study
In September 1944, as a result of the Dutch resistance against the Nazi occupation, the Nazis restricted food into the Netherlands, leading to a famine that ended up killing more than 20,000 people before the liberation of the country. This period of Dutch history is known as the Dutch Hunger Winter, and it has led to some very significant evidence of the role of epigenetics in health.
Tobi et al (2018) published a study that is based on almost 30 years of research. What fascinated the researchers was that children born to women that were pregnant during the famine when they reached middle age had higher levels of triglycerides and LDL cholesterol. They also experienced higher rates of such conditions as obesity, diabetes, and schizophrenia. Why might this be true?
The researchers started by taking blood samples from 422 adults who had been in the womb during their famine and 463 siblings that served as controls. From the blood samples, they were able to analyze the DNA. They also gathered data regarding the health of the participants, including BMI. The results were that they found evidence of gene expression that may be linked to higher BMI. The researchers propose that the Dutch Hunger Winter added a methyl group to fetuses born to starving mothers, which made the PIM3 gene (responsible for raising metabolism) less active — and continued to do so for life.
There have been many different studies carried out on these children. You can see more about the research on the health effects of the famine in this video with researcher Tessa Roseboom.
- Research can be carried out on animals due to their anatomical and genetic similarities to humans.
- Several studies are experimental making it possible to replicate the findings to test for reliability - and to potentially establish a cause and effect relationship.
- The biological approach is highly reliant on animal models. There are doubts as to whether the research done on animals can actually be generalized to humans.
- There are ethical concerns about the use of OB mice.
- It is impossible to rule out the role of environmental factors in genetic research.
- Many of the experiments look solely at eating behaviour and do not necessarily then predict that this will lead to obesity.

 IB Docs (2) Team
IB Docs (2) Team
