16.1 Rate expression & reaction mechanism
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 6.2 Rate expression & reaction mechanism. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)
 Learning outcomes
Learning outcomes
.png) After studying this topic you should be able to:
After studying this topic you should be able to:
Understand
- Reactions may involve more than one step and the slowest step determines the rate of reaction (This step is known as the rate determining step, RDS.
- The number of reactant particles taking part in any specified step is known as the molecularity.
- The order of a reaction can be either an integer or fractional. The order of a reaction can describe, with respect to a reactant, the number of particles
taking part in the rate-determining step. - All rate equations can only be determined by experiment.
- The units of a rate constant are determined by the overall order of the reaction and the value of the rate constant, k, is affected by temperature.
- Catalysts alter a reaction mechanism by producing a step with a lower activation energy.
Apply your knowledge to:
- Deduce the rate expression for an equation from experimental data and solve problems involving the rate expression.
- Sketch, identify, and analyse graphical representations for zero, first and second order reactions.
- Evaluate proposed reaction mechanisms to show they are (or are not) consistent with kinetic and
stoichiometric data.
Relationships & vocabulary
Nature of science
Rather like 6.1 Collision theory & rates of reaction, this sub-topic also provides a good example of the Principle of Occam’s razor. As theories develop they need to remain as simple as possible while maximizing their powers of explanation. Because the probability of collisions between three reacting species is low it means that stepwise reaction mechanisms are more likely.
International-mindedness
The first industrial catalyst to be used was for the Contact process to produce sulfuric acid. It has been stated that for a long time the amount of sulfuric acid produced by a country closely mirrored the country’s economic health. What are some of the current indicators for a country’s economic health?
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 6 & 16: Chemical kinetics.
Vocabulary
| rate constant, k | rate expression | rate equation | order of reaction | overall order |
| half-life, t½ | slow step | fast step | rate-determining step | reaction mechanism |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
1. While I was searching around on the Internet I came across a worksheet with questions on the rate expression.
The first two questions are:
1) Write the following for the reaction N2 + 3H2 → 2NH3
(a) The rate expression for the reaction
(b) The order of the reaction in each of the reagents
(c) The overall order of the reaction
2) The rate constant for the reaction HNO3 + NH3 → NH4NO3 is 14.5 L / mol.sec.
If the concentration of nitric acid is 0.050 M and the concentration of ammonia is 0.10 M, what will the rate of this reaction be?
The answers are then given:
1).
(a) Rate = k[N2][H2]3
(b) The reaction is first order in nitrogen and third order in hydrogen.
(c) The overall order of the reaction is fourth order
2). Rate = k[HNO3][NH3]
Rate = (14.5 L / mol.sec)(0.050 M)(0.10 M)
Rate = 0.073 mol / L. sec
The question uses M and L /mol. sec whereas we use mol dm-3 and dm-3 mol -1 s-1 and no states are given and k is not in italics but all that is only a question of convention. What is completely wrong is that these questions are unanswerable with the information given. Rate expressions cannot be determined from the stoichiometric equation. They can only be arrived at from experimental data.
The rate equation for the first question can only be expressed as:
rate = k[N2]x[H2]y
where x and y are the order of the reaction with respect to nitrogen and hydrogen and must be found by experiment.

In fact work by Ertl and others have shown that in the Haber Process (right) where an iron catalyst is used the reaction is complex and thought to proceed by the following steps:
- N2 (g) → N2 (adsorbed)
- N2 (adsorbed) → 2 N (adsorbed)
- H2(g) → H2 (adsorbed)
- H2 (adsorbed) → 2 H (adsorbed)
- N (adsorbed) + 3 H(adsorbed)→ NH3 (adsorbed)
- NH3 (adsorbed) → NH3 (g)
The second step is the slowest step which suggests that [H2] does not even appear in the rate equation.
Another example to illustrate the same point is the reaction of hydrogen with halogens:
H2(g) + X2(g) → 2HX(g)
When X is iodine, the experimentally determined rate expression looks as if it could be deduced from the stoichiometric equation as it is:
rate = k[H2(g)][l2(g)]
but it is very different case when bromine is involved
rate = k[H2(g)][Br2(g)]½ / ([Br2(g)] + k'[HBr(g)]
2. Just a question concerning reaction mechanisms that a student of mine once asked which you might like to reflect upon.
We were looking at nucleophilic substitution reactions and specifically why the hydroxide ion is a better nucleophile than water. During the discussion I confirmed the answer given by most of the students that the hydroxide ion is more electron rich and therefore it will be attracted more strongly to the δ+ carbon atom of the halogenoalkane. Her question was: How can we know that hydroxide ions are better nucleophiles than water in the case of tertiary halogenoalkanes? She argued that when the halogenoalkane is tertiary the first step in the reaction is the dissociation of the halogenoalkane into the halogen ion and the tertiary carbocation. Since this is a first order reaction with the rate only depending upon the concentration of the halogenoalkane the first step is the slow step in the reaction. Therefore the nucleophile will play no part in the overall rate and so there should be no difference in the rate when either water or hydroxide ions are used as the nucleophile.
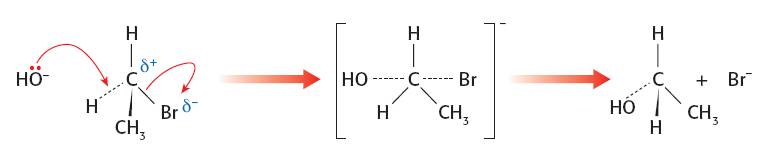
SN2 mechanism. The better nucleophile should give a faster rate as both the nucleophile and the halogenoalkane are involved in the rate determining step:
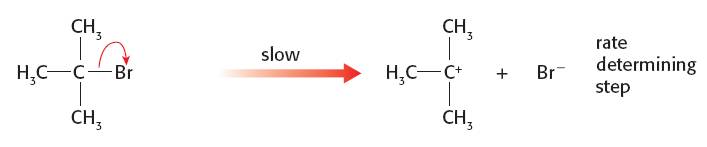
SN1 mechanism. The first step is the slow step with only the halogenoalkane involved:
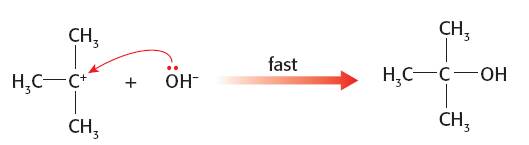
Since the second step is faster the nature of the nucleophile should make no difference to the overall rate of the reaction so it is impossible to determine which is the better nucleophile in this situation:
She was a smart student!
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MCTest: Rate expression & reaction mechanisms.
For short-answer questions on rate expressions see Rate expression questions.
For short-answer questions on reaction mechanisms see Reaction mechanisms questions.
More resources
1. Deducing the rate expression by looking at initial rates by Richard Thornley, an IB teacher at the International School of Genoa. The video also looks at the units of the rate constant, k, although this is explained more in the next video.
2. Another Richard Thornley video - this time on order or reaction and rate constants.
3. A nice visual demonstration by Richard Thornley showing the importance of the rate determining step.
4. There are a lot of 'lecture' type video clips on YouTube on reaction mechanisms. Here is one by Mark Rosengarten.

 IB Docs (2) Team
IB Docs (2) Team 




























