Introduction to materials science

 A.1 Introduction to materials science (1.5 hours)
A.1 Introduction to materials science (1.5 hours)
Pause for thought
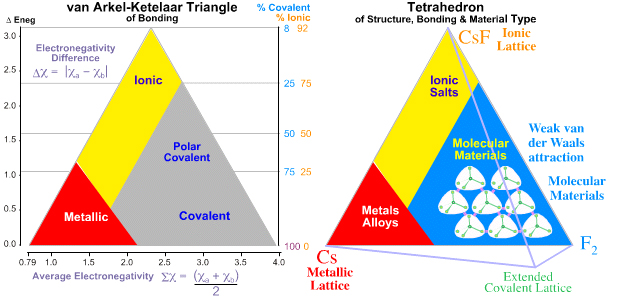
It is the first time that triangular bonding diagrams have appeared on the IB syllabus, although they have been on the Cambridge pre-U syllabus (another international examination for 16-19 year olds) for several years. I’m not convinced as to how useful they are. To me they do not really go much further than the old rule of thumb that if the electronegativity difference is greater than about 1.8 the compound is likely to be ionic. They remind me a bit of the octet rule. Both work for simple compounds but both are based on dubious assumptions and fail for more complex examples. Even if we can deduce the percentage of covalent to ionic bonding in a compound, does that really help to understand the properties? Metallic, covalent and ionic are nice labels and ways of classifying materials but their properties can only be generalised. For example metals “have high boiling points” but not all metals do – mercury is a liquid with an appreciable vapour pressure at room temperature. ‘‘Covalent substances have lower boiling points than ionic substances”– try telling that to diamond and sodium chloride!
One of the problems is electronegativity itself. It is not an absolute value and indeed there are different scales of electronegativity. The values are arrived at by considering how a bonding pair of electrons is shared between the two bonding atoms so in a sense the percentage of covalent and ionic bonding has already been taken into account. My two biggest criticisms though are:
1. In the triangular diagram given in Section 29, ‘covalent’ only applies to simple covalent molecules and does not consider macromolecules like diamond, silica or graphite. Diamond and graphite both appear in the covalent section but what new knowledge is gained from that information? It does not enable us to deduce that one conducts electricity and one does not. The tetrahedral diagram (above right), which is not on the syllabus, does attempt to address this.
2. It does not distinguish between different oxidation states. Consider the chloride of a metal M, where M has an electronegativity value of 1.8. Chlorine’s value is 3.2 so the difference is 1.4 and the average is 2.5. This puts the chloride of M in the polar covalent region so we might conclude that it will not conduct electricity when molten and will have a reasonably high boiling point although lower than that of an ionic compound. The problem is that M is lead and lead forms two chlorides, lead(II) chloride, PbCl2, and lead(IV) chloride, PbCl4. Lead(II) chloride is ionic, conducts electricity when molten and boils at 950 oC, Lead(IV) chloride is a yellow non-conducting covalent oily liquid at room temperature and boils at 50 oC. The van Arkel – Ketelaar diagram is of absolutely no use whatsoever in deducing this information.
Nature of Science
In the past some materials were used for different purposes than they are today. This is due to improvements in technology and the increasing scientific understanding of their properties.
History has been characterized by the materials civilisations have developed and used, e.g. Stone Age, Bronze Age and Iron Age. There are different ways of classifying materials according to desired patterns.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesPermeability to moisture should be considered with respect to bonding and simple packing arrangements. The properties of metals, polymers and ceramics should be considered in terms of metallic, covalent, and ionic bonding. A triangular bonding diagram (van Arkel-Ketelaar diagram) can be found in Section 29 of the data booklet. International-mindednessConsider the materials used by ancient civilizations, e.g. the Aztecs, Romans, and Chinese. The materials they used were similar, even though these ancient civilizations were located in very different parts of the world. |
Teaching tipsThis first introductory topic really builds upon the core topic on bonding, particularly 4.4 Intermolecular forces and 4.5 Metallic bonding . Essentially what you will need to ensure is that students understand how to use the triangular bonding diagram in Section 29 of the data booklet in order to deduce the properties of a material (in as much as it works – see Pause for thought above!) and then be able to relate the different properties of materials to the type of bonding. By necessity, many of these can only be generalisations, but include those listed (melting point, permeability, conductivity, elasticity, brittleness) and also boiling point and malleability. Note that there are no chemical properties listed even though metals tend to form basic or amphoteric oxides and non-metal oxides tend to be acidic or neutral. When looking at the different names given to ancient civilisations stress that they were mainly based on the type of materials used to make tools and that the discovery of the metals (or alloys) used tends to follow their position in the reactivity series, as that is related to the ease with which they can be obtained from their ores. Composites are important. The first to be used was probably mud and straw to make bricks. Reinforced concrete is a good example and modern composites, including glass and carbon fibres and epoxy resins, have a multitude of uses (even military - the Humvee was the first all-composite military vehicle). However there is not really much detail that can be given except the syllabus statement that they are ‘composed of two distinct phases, a reinforcing phase that is embedded in a matrix phase’ which makes them stronger than either of their components. | Study guide
Page 110 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Introduction to materials science. For short-answer questions see Materials science introduction questions together with the worked answers on a separate page Materials science introduction answers. Vocabulary listtriangular bonding diagram |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A demonstration of some of the physical properties of metals, such as tensile strength, elasticity, malleability and melting point.
2. A good introduction to composites and their properties by BioNetwork.
3. An excellent video describing and explaining how van Arkel- Ketelaar triangular bonding diagrams work. The discussion then goes further to look at tetrahedral bonding diagrams. It will give your students a really good understanding of how it all works.
4. Richard Thornley gives a simple animated video of van Arkel-Ketelaar diagrams specifically for the IB.

 IB Docs (2) Team
IB Docs (2) Team 























