Preparation & purification of aspirin

 Introduction
Introduction

The photograph of the packet above is an interesting image as each tablet contains 75 mg of aspirin. When aspirin is taken to relieve the symptoms of pain or fever the tablets usually contain 300 mg of aspirin. The 75 mg tablets are commonly prescribed to be taken once a day by people suffering from hypertension (high blood pressure). Because aspirin has the effect of thinning the blood this helps to reduce the risk of stroke or heart attack. This point is specifically mentioned in 'Understandings' listed in D.2 Aspirin & penicillin in Option D: Medicinal chemistry.
Teacher's notes
This experiment is a good way to cover both some practical skills and the theory of aspirin synthesis that forms part of sub-topic D.2 Aspirin & penicillin in Option D: Medicinal chemistry. Students enjoy making a drug that is on the syllabus (and legal to make in a school laboratory!). It could also be given to students who are not studying Medicinal chemistry as an option because it is a good example of recrystallisation and the use of melting point as a way of testing the purity of a product.
Students should be familiar with esterification by reacting alcohols with carboxylic acids as this is covered in sub-topic 10.2: Functional group chemistry. However -OCOR is a better leaving group than -OH and consequently acid anhydrides are much more reactive with nucleophiles than carboxylic acids. For those studying Medicinal chemistry you can relate this to the diesterification reaction that must occur in order to convert morphine, a strong analgesic, into heroin which is an even stronger analgesic. Morphine is an alcohol and it is more efficient to react one mole of morphine with two moles of ethanoic anhydride than with two moles of ethanoic acid in order to convert it into heroin.
If students have already done the experiment to Analyse aspirin tablets then they may also like to analyse the aspirin they prepare in this experiment to show how pure their product is. They can also determine the melting point of their product to determine its purity1,2. Alternatively, or in addition, they could perform thin layer chromatography and develop the chromatogram in iodine (or under a UV lamp) to show whether there is just one component in their product or whether another component such as unreacted 2-hydroxybenzoic acid) also shows up. The syllabus mentions the use of infrared spectroscopy and I have provided a question on this at the end of the practical sheet.
Although it is a rather straightforward practical there is much good chemistry in this experiment. There are no real hazards although of course care will be needed when handling concentrated sulfuric acid and the ethanoic acid and ethanoic anhydride. The only thing to warn students is that sometimes the reaction goes quite fast and the reaction vessel can get very hot. There is a rather bad video showing you how reactive this can get. Note the safety glasses on the bench and the girl who is not wearing any eye protection.
Explosion during aspirin preparation ![]()
Footnotes
1 Actually the melting point of aspirin is quite a nice example of the Nature of Science. You would think that the value would be clearly stated in data books and yet there is considerable disagreement. The value often quoted is 134-136 oC with decomposition. Some sources however quote 138-140 oC whereas others give exactly 135 oC.
2 The Royal Society of Chemistry has produced a really good resource for both teachers and students on the synthesis of aspirin which also includes how to test for its purity.

 Student worksheet
Student worksheet
PREPARATION AND PURIFICATION OF ASPIRIN
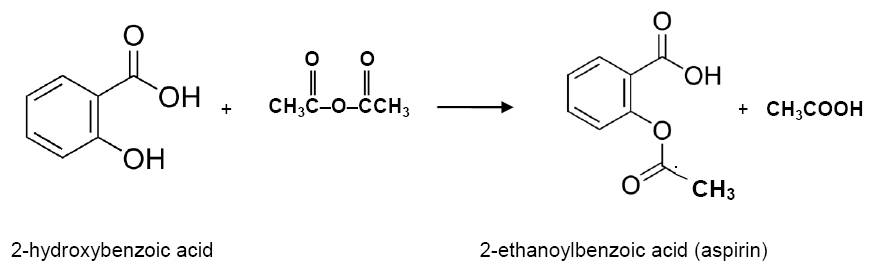
The aim of this experiment is to synthesise a pure sample of aspirin and test the product to see how pure it is. The preparation is relatively simple but through the practical you will use the techniques of recrystallisation and the use of the melting point apparatus. The synthesis is basically esterification but instead of reacting a carboxylic acid with the phenol (alcohol) group of the 2-hydroxybenzoic acid an acid anhydride is used in its place. Note that on the syllabus the IB uses the common name 'salicylic acid' for 2-hydroxybenzoic acid.
The equation for the reaction is:
ENVIRONMENTAL CARE:
The organic chemicals used are quickly broken down in the environment and therefore present no threat.
SAFETY:
Ethanoic anhydride, 'glacial' ethanoic acid and concentrated sulfuric acid are all corrosive. Treat them with care and wear an apron and safety goggles throughout the experiment.
PROCEDURE:
Shake together 2.0 g of 2-hydroxybenzoic acid (salicylic acid) with 4 cm3 of ethanoic anhydride in a 100 cm3 conical flask. Now add 5 drops of concentrated sulfuric acid and continue agitating the flask. It may become quite warm for a while. Crystals of aspirin will appear and soon the mixture will form into a crystalline mass. Now add 4 cm3 of cold 'glacial' ethanoic acid, and cool the flask in a beaker containing a mixture of crushed ice and water. Filter off the aspirin crystals onto a small filter, and wash once with ice water in order to remove residual acid. Recrystallise the aspirin, by dissolving the crystals in a small quantity of hot water, and allow to cool. A mass of very pure aspirin crystals should form. Filter them again, and leave overnight on a watch glass to dry completely.
Weigh the mass of pure dry aspirin and calculate the yield as a percentage of the theoretical maximum value. This may well be considerably below 100% because you have carried out a recrystallisation, and have worked on a very small scale. Both lead to significant losses of material. In addition, many organic reactions either do not go to completion, or produce by-products. Using the melting point apparatus determine the melting point of your sample and compare it with the data book value. If you have time you can further test the purity of your sample by a titration as described in another practical - Analysis of aspirin tablets.
QUESTIONS:
1. Methyl salicylate is a different ester that can also be prepared from 2-hydroxybenzoic acid. Discuss how this could be prepared and deduce the equation for the reaction.
2. The infrared spectrum of pure aspirin is shown below: (Taken from G.Neuss, Oxford IB Chemistry Study Guide, 2014, OUP)
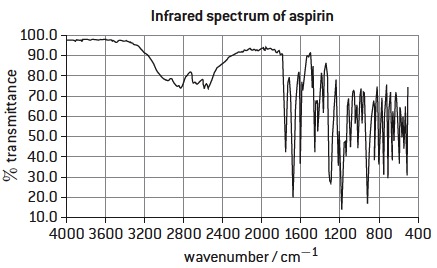
Explain why there are two peaks in the region 1700 -1750 cm-1.
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team