15.1 Energy cycles
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 15.1 Energy cycles. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand
Understand
- Equations, such as M+(g) → M+(aq) can be used for enthalpy/energy of hydration, ionization, atomization, electron affinity, lattice, covalent bond and solution.
- The lattice enthalpy, enthalpy of solution, and the hydration energy can all be related in an energy cycle.
Apply your knowledge to:
- Construct Born-Haber cycles for group 1 and 2 oxides and chlorides.
- Construct of energy cycles from hydration, lattice and solution enthalpy. Examples could include the dissolution of solid sodium hydroxide, NaOH(s), or ammonium chloride in water, NH4Cl(s), in water.
- Calculate enthalpy changes using Born-Haber or dissolution energy cycles.
- Relate the magnitude of lattice and hydration enthalpies to the size and charge of ions.
- Carry out laboratory experiments which might include single replacement reactions in aqueous solutions.
Relationships & vocabulary
Nature of science
Energy cycles permit the calculation of values that cannot be determined directly. This relates to the importance of replicating quantitative measurements to ensure reliability.
International-mindedness
The significance of being able to obtain indirect measurements using energy cycles for things that cannot be measured directly is of global importance. For example, snow depth cover, borehole temperatures, glacier recession, rates of evaporation and precipitation cycles are among some indirect indicators of global warming. Because the factors that affect global warming are no respecters of international boundaries it is important for countries to collaborate to combat problems like global warming?
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 5 & 15: Energetics/thermochemistry.
Vocabulary
| Born-Haber cycle | lattice enthalpy | enthalpy of atomization |
| electron affinity | enthalpy of solution | enthalpy of hydration |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
The idea of using an energy cycle to solve problems involving Hess's Law has already been introduced in sub-topic 5.2 Hess's Law. This Higher Level sub-topic involves the use of energy cycles that contain the lattice enthalpy and or enthalpies of hydration and solution. A Born-Haber cycle is simply a special case of Hess’s Law. It refers to the enthalpy change of formation of an ionic compound. It can either proceed directly or in a series of separate steps which involve several different enthalpies changes which assume that the salt formed is 100% ionic. Most of these enthalpy changes can be measured experimentally so the purpose of the Born-Haber cycle is either to find an unknown enthalpy value or to compare the experimental or theoretical value with that determined by the cycle. For the IB this usually means determining the value for the lattice enthalpy. If there is good agreement between the experimental and theoretical lattice enthalpy which has been calculated assuming a purely ionic structure then it is reasonable to assume the compound is close to being 100% ionic. However if the two values do not agree then it suggests that the salt contains some degree of covalent character. There were problems with this on the last programme as the theoretical values in the then data booklet had already taken into account any covalent character. The current data booklet for first exams in 2016 does not contain the theoretical values and in the guidance notes to teachers it states that the polarising effect of some ions which produces covalent character in some essentially ionic substances will not be assessed.
A Born-Haber cycle should be exactly that - a cycle. Many text books and websites actually give it as an enthalpy level diagram. If we take sodium chloride as the classic example then the basic reaction is the reaction of one mole of solid sodium metal with half a mole of chlorine gas to give one mole of solid sodium chloride. This can either proceed directly or via the gaseous atoms and ions as shown below:
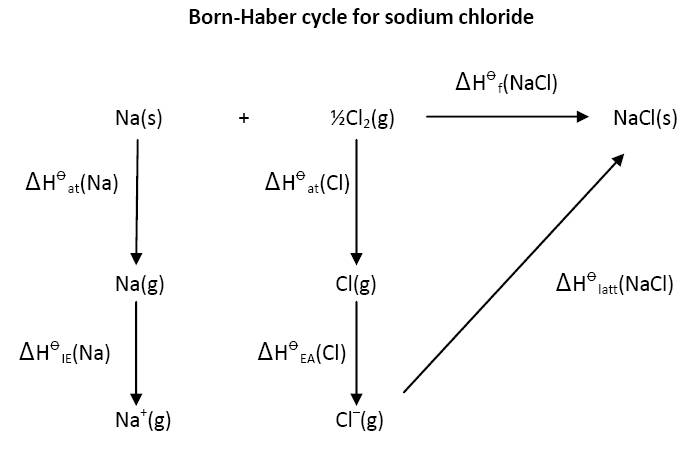
The great strength of the cycle is that is does not matter whether the values are positive or negative (or their quantitative value). It is clear that
∆H![]() f(NaCl) = ∆H
f(NaCl) = ∆H![]() at(Na) + ∆H
at(Na) + ∆H![]() at(Cl) + ∆H
at(Cl) + ∆H![]() IE(Na) + ∆H
IE(Na) + ∆H![]() EA(Cl) + ∆H
EA(Cl) + ∆H![]() latt(NaCl)
latt(NaCl)
Contrast this with the enthalpy diagrams (below) for sodium chloride and calcium oxide. You need to know both the size and the signs of the values of the enthalpy changes (which in the case of electron affinities can be different) before you can draw the diagram correctly. To me the relationship between all the values also seems less obvious. The IB of course accepts either cycles or enthalpy diagrams as correct.


Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Energy cycles.
For short-answer questions see Energy cycles questions.
More resources
1. A straightforward video showing how a Born-Haber cycle for magnesium chloride can be used to find the value for the electron affinity of chlorine.
2. Lattice enthalpy and electron affinity specifically for the IB by Richard Thornley.
3. Factors (radii, charge and charge density) affecting the size of the lattice enthalpy - also by Richard Thornley.

 IB Docs (2) Team
IB Docs (2) Team 






















