Stereochemistry in biomolecules
 B.10 Stereochemistry in biomolecules (2 hours)
B.10 Stereochemistry in biomolecules (2 hours)
Pause for thought
The syllabus states clearly that students need to understand that with one exception, amino acids are chiral, and only the L-configuration is found in proteins.
Chirality is no problem but if students are to understand that only the L-configuration is found in nature then they need to be able to distinguish between the L- and D- forms of 2-amino acids. The syllabus does specifically require students to be able to distinguish between the L- and D- forms of sugars such as glucose and fructose and gives clear details on how to do this. Similarly it explains how to distinguish between the α and β forms of cyclic sugars. However no mention is made under ‘Applications and skills’ of using the CORN rule for 2-amino acids.
The rule is actually quite simple. The molecule needs to be positioned so that the hydrogen atom is pointing away from the viewer. This brings the other three groups to the front. If the order going clockwise is –COOH, -R, -NH2 then it is the D- enantiomer and if the CORN order is going anticlockwise then it is the L- isomer.
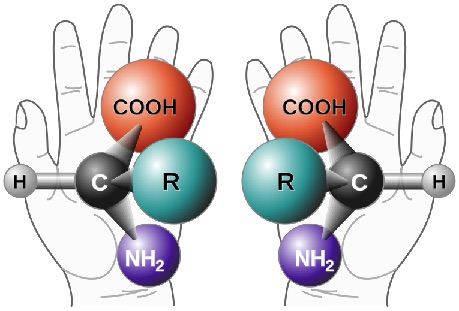
Left hand CORN is anticlockwise so L-configuration, right hand CORN is clockwise so D-configuration.
(Image from Wikimedia commons)
It really is not at all clear whether students need to know this but how else can they distinguish between the L- and D- forms of 2-amino acids?
Nature of Science
The biological activity of chiral molecules is often specific to just one enantiomer. Chemical reactions involving a chiral environment provide a guiding distinction between living and non-living matter.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe names of the enzymes involved in the visual cycle are not required. The relative melting points of saturated and cis-/trans-unsaturated fats should be understood. International-mindednessThe standards of food labelling with respect to its chemical content, including the type of fats present, can vary greatly from country to country. |
Teaching tipsThis sub-topic is essentially concerned with chirality in amino acids and sugars and cis-/trans- isomerism in unsaturated fats and carotenoids. It very much builds upon the content covered in B4: Carbohydrates and if you are fortunate enough to be teaching Higher Level students on their own (rather than in a mixed class with Standard Level students present too) then it makes sense to combine this sub-topic with B4 and teach both together. Amino acids have been introduced in sub-topic B3: Proteins & enzymes and chirality in sub-topic 20.3 Stereochemistry so it should be obvious to students that all 2-amino acids (apart from glycine) can exist as enantiomers. Read the ‘Pause for thought’ I have written in B4 as it mentions D and L sugars and how to distinguish between the α and β forms of glucose but raises the problem about how to distinguish between the D and L forms of 2-amino acids. You will need to extend the condensation reactions of sugars to cover the structures of cellulose and starch. Starch involves the condensation of α-D-glucose and can be digested by humans. Amylose has α-1,4-glycosidic bonds and amylopectin contains both α-1,4-glycosidic bonds and α-1,6-glycosidic bonds. Cellulose is formed from β -D-glucose with β -1,4- linkages. It cannot be digested by humans but plays an important role in diet as roughage. Explain why cis-unsaturated fats have lower melting points than the corresponding saturated fats due to the ‘kink’ formed in the chain so they cannot pack so closely. Trans- unsaturated fats, which can be formed during the industrial partial hydrogenation of cis- unsaturated fats, are straighter than the cis- isomer so have higher melting points than their cis- counterparts (although still lower than the corresponding saturated fat). Trans- fats are harder to metabolise and excrete so accumulate in fatty tissue and are thought to increase the amount of low-density cholesterol (LDL), which can lead to cardiovascular disease. The visual cycle involves the protein opsin and the covalently bonded co-factor retinal, which is produced in the retina from vitamin A. When light falls on the retina it converts the retinal from the cis- to the trans- form and a nerve impulse is transmitted via an interaction which causes a conformational change in the structure of opsin to send a signal along the optic nerve to the brain. | Study guide
Page 139 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Stereochemistry in biomolecules. For short-answer questions see Stereochemistry in biomolecules questions together with the worked answers on a separate page Stereochemistry in biomolecules answers. Vocabulary liststarch |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A very simple, but to the point, video by Bite Sci-zed on saturated and cis- and trans- unsaturated fats describing what they are and the factors affecting their melting points.
2. Fischer projections. How to distinguish between the D- and L- forms of sugars.

 IB Docs (2) Team
IB Docs (2) Team 



















