Superconducting metals & X-ray crystallography
 A.8 Superconducting metals & X-ray crystallography
A.8 Superconducting metals & X-ray crystallography
(5 hours)
Pause for thought
In JIB Docs (2) Teamary 2017 yet another revised version of the data booklet became available. However there is still a real problem with the atomic radii given for the elements in Groups 1, 2 and 13.
The images below show how the values for Groups 1 and 2 have changed from the data booklet used for the previous programme.
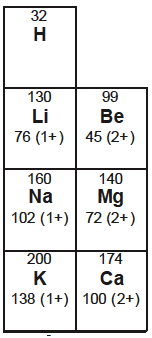
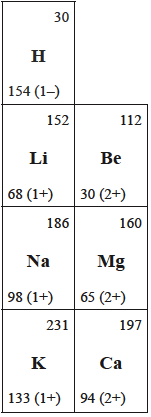
The table on the left is from Section 9 of the current 2016 data booklet whereas the table on the right is from Table 8 of the 2009 data booklet. Both give the atomic and ionic radii when multiplied by 1 x 10−12 m.
‘Under the Applications and skills’ section for this sub-topic it states “Determination of the density of a pure metal from its atomic radii and crystal packing structure”.
Consider the following problem that could easily be set by the IB to cover this area.
Sodium forms a body-centred cubic unit cell. That is, the atoms occupy 68% of the available space. Determine the density of sodium.
To solve this problem we would use the 2016 data booklet (latest version updated in JIB Docs (2) Teamary 2017) to find that the radius of one sodium atom is 1.60 x 10-10 m. We would then find the volume of one atom and hence the volume of one mole of sodium atoms. Knowing that the packing is only 68% efficient we could find the actual volume of a lattice containing one mole of sodium atoms. We know from Section 6 in the data booklet that this has a mass of 22.99 g mol-1 so we can then determine the density by dividing the mass by the volume.
The volume of one sodium atom = 4/3 πr3
= 4/3 x 3.14 x (1.60 x 10-10)3 m3 = 1.715 x 10-29 m3
The volume of one mole of sodium atoms
= 1.715 x 10-29 x 6.02 x 1023 m3 = 1.032 x 10-5 m3
The volume of a lattice containing one mole of sodium atoms
= 100/68 x 1.032 x 10-5 m3 = 1.518 x 10-5 m3
The density of sodium (knowing that the atomic mass of sodium is 22.99 g mol-1)
= 22.99 / (1.518 x 10-5) g m-3 = 1.51 x 106 g m-3 = 1.51 g cm-3
There is a serious problem with this answer. Students should realise that it cannot possibly be correct as sodium floats on water so it has a density of less than 1 g cm-3.
In fact the density of sodium is 0.968 g cm-3 (or 9.68 x 105 g m-3)
What happens if we use this value and work backwards to find the radius of a sodium atom?
Volume of lattice containing one mole
= 22.99 / (9.68 x 105) m3= 2.38 x 10-5 m3
Volume of one mole of sodium atoms
= 68/100 x 2.38 x 10-5 m3 = 1.62 x 10-5 m3
Volume of one sodium atom
= 1.62 x 10-5 / 6.02 x 1023 m3 = 2.69 x 10-29 m3
(Radius of a sodium atom)3 = 2.69 x 10-29 x ¾ x 1/3.14 = 6.43 x 10-30 m3
Radius of one sodium atom
= (6.43 x 10-30)⅓ m = 1.86 x 10-10 m = 186 x 10-12 m
Surprise surprise. Where have we seen this value before? It is the value given in the old data booklet (see above) that used the value for the metallic radius for sodium (and the other metals). I do wonder why all the values were changed and whether anyone gave any serious thought to it. The new values, which give the covalent radii rather than the metallic radii, have the potential to cause considerable problems in the examinations on the new programme when the new data booklet (for first exams in 2016) will be used.
Nature of science
The discovery that superconducting materials have zero electrical resistance below a certain temperature, provides a good example of how theories need to be modified to fit new data.
This sub-topic also illustrates the importance of understanding the basic scientific principles behind modern instrumentation.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesOnly a simple explanation of the Bardeen-Cooper-Schrieffer (BCS) theory with Cooper pairs is needed. At low temperatures a passing electron distorts the positive ions in the lattice. A second electron is attracted to this slight positive deformation leading to a coupling of these two electrons. The operating principles of X-ray crystallography are not required. Only pure metals with simple cubic cells, body centred cubic cells (BCC) and face centred cubic cells, (FCC) should be covered. Although the perovskite crystalline structures of many superconductors can be analysed by X-ray crystallography these will not be assessed. The application of the Bragg's equation is limited to simple cubic structures. International-mindednessAdvanced analytical techniques have many applications (e.g. in forensics, mineral exploration, medicine etc.) It is worth considering how world economies are affected by unequal access to these advanced techniques. |
Teaching tipsThis is another sub-topic where there is considerable overlap with physics and it may be worth talking with your physics colleagues about their approach to teaching superconductivity. In a sense there are two separate topics contained within this sub-topic - superconductivity and X-ray crystallography. The syllabus coverage of superconductivity is confined pretty much to a simple account. Describe what it is and how BCS theory describes how electrons are able to flow freely in pairs through superconductors. Students will need to be familiar with the two basic shapes shown by graphs of resistance plotted against temperature for types 1 and 2 superconductors and be able to interpret them. The more difficult part of this sub-topic to teach is the use of the Bragg equation and its application to crystal structures. Because X-ray crystallography is mentioned briefly in Topic 21.1 Spectroscopic identification of organic compounds (HL) I actually introduce the Bragg equation there as it helps to explain how X-rays can be used to deduce a structure. For this sub-topic you will need to explain about unit cells and the fact that although there are eight atoms contributing to a simple cubic unit cell, the number of atoms making up the cell is only one as each atom contributes to seven other cells so only contributes 1/8th to a single unit cell etc. You will also need to explain why the coordination number changes for the different types of cubic unit cell. The Bragg equation can be used to manipulate angles of incident waves, wavelengths and the distances between atoms and then give examples for calculating the density of Group 1 or Group 2 metals once their metallic radius and unit cell type is known. Emphasise that because the radius is cubed in density calculations a small difference in radius can make a big difference to the calculated result. | Study GuidePages 107 & 119 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Superconducting metals & X-ray crystallography. For short-answer questions see Superconducting metals & X-ray crystallography questions together with the worked answers on a separate page Superconducting metals & X-ray crystallography answers. Vocabulary listsuperconductor/superconductivity |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. An explanation of superconductivity at about the right level (it is actually for British A level Physics).
2. A very neat demonstration of the Meissner effect - although unfortunately the sound explanation is not present.
3. Richard Thornley applies the Bragg equation. However remember that although the theory is fine the radii used from the data booklet are the covalent radii not the metallic radii so some of values are not correct (see 'Pause for thought above').

 IB Docs (2) Team
IB Docs (2) Team 























