Nanotechnology

 A.6 Nanotechnology (2 hours)
A.6 Nanotechnology (2 hours)
Pause for thought
You might be forgiven for thinking that this sub-topic is more concerned with physics rather than chemistry. This is supported by the fact that Andre Geim and Konstantin Novoselov, both from the University of Manchester, UK, who were awarded the Nobel Prize in 2010 “for ground-breaking experiments regarding the two-dimensional material graphene” won the prize for physics not chemistry.
They obtained graphene which is only one carbon atom thick using just a piece of adhesive tape. Graphene is one of the most promising materials in nanotechnology. It has remarkable properties. For instance, it conducts electricity as well as copper and is the best conductor of heat of all known materials. Although it is completely transparent, it is so dense that not even single atoms of helium gas can pass through it. When mixed with plastics it can turn them into electrical conductors that are thin, lightweight and elastic. Such composites might be used to build aeroplanes, cars and satellites in the future. In chemistry catalysts involving layers of graphene have been shown to highly efficient as they provide the ultimate model of catalytic support in terms of available surface area. One such example, shown in the diagram below, shows how nanoparticles of rhodium and nickel embedded in a mesh of graphene can catalyse the decomposition of hydrazine into nitrogen and hydrogen.
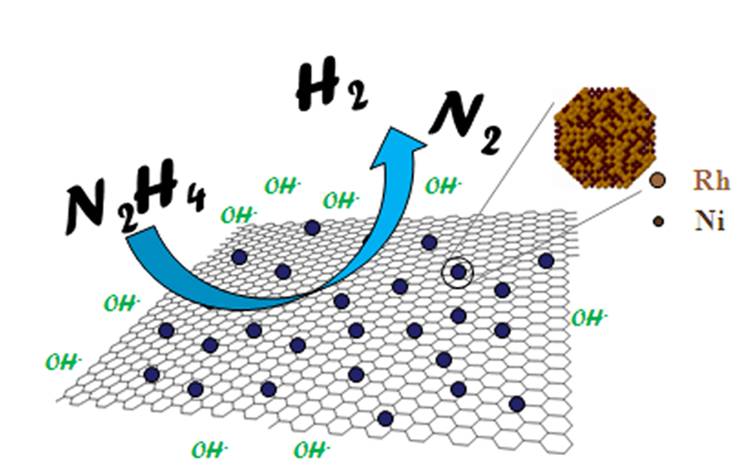
Representation of the catalytic decomposition of hydrazine using Rh/Ni nanoparticles on graphene (image from Zhang Group)
Nature of Science
The study of positioning of atoms had been enabled by the development of high power electron microscopy.
The role of trial and error has been central to the development of theories in nanotechnology.
“The principles of physics, as far as I can see, do not speak against the possibility of manoeuvring things atom by atom. It is not an attempt to violate any laws; it is something, in principle, that can be done; but in practice, it has not been done because we are too big.”
— Richard Feynman, winner of the Nobel Prize in Physics 1965.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesPossible implications of nanotechnology include: The electrical conductivity of graphene and fullerenes can be explained in terms of delocalization of electrons. Explanations based on hybridization are not required. International-mindednessResearch has shown that inhaling nanoparticle dust may be as harmful as asbestos. Should there be international legislation to regulate nanotechnology or would that hinder research? What are the implications of nanotechnology for space exploration? |
Teaching tipsI doubt if anyone teaching this underestimates the importance of nanotechnology and the reasons for including this sub-topic on the syllabus. Unfortunately the current syllabus content tends to be rather factual and places the emphasis more on recalling how carbon nanotubes can be made rather than focusing more on their uses and potential. Explain first what range is covered by the term 'nanoscale' and how nanotechnology can be approached from a 'bottom-up' approach whereby the individual atoms are used to build materials or from the 'top-down' approach where nanomaterials are obtained by breaking down larger entities. Give the facts for arc discharge, CVD and HIPCO stressing the relevant equations and reactions and explain how the properties of nanotubes depend upon the delocalisation of electrons, their high tensile strength and high surface area to volume ratio. There are many articles published on the uses (both actual and potential) of nanotechnology. It may be useful to get your students to do some of their own research to find suitable examples to share. Similarly the potential dangers of nanoparticles are well documented. These are due largely to their small size although at the moment the actual dangers do not appear to have been quantified. They may also cause some social and economic problems if societies currently dependent upon established technologies fail to adapt. | Study guide
Page 117 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Nanotechnology. For short-answer questions see Nanotechnology questions together with the worked answers on a separate page Nanotechnology answers. Vocabulary listnanoscale |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. Synthesising graphene using chemical vapour deposition - a short lecture from the University of Barcelona.
2. A longer (20 minutes) TED talk by Professor Mikael Fogelström on the possibilities of graphene technology. Well worth letting students watch this in their own time.

 IB Docs (2) Team
IB Docs (2) Team 











