Reaction mechanisms questions
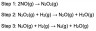 Assignment:
Assignment: ![]() Questions on Topic 16.1: Reaction mechanisms
Questions on Topic 16.1: Reaction mechanisms
This page of questions can be marked as direct student access either for assigning as a test or for students to work on in their own time. If you do not wish to use student access, links to downloadable versions of the questions and, separately the worked answers, can be found at Printable versions of written tasks.
Hydrogen and nitrogen(II) oxide react to form nitrogen and steam according to the following equation:
2H2(g) + 2NO(g) → N2(g) + 2H2O(g)
The experimentally determined rate equation for this reaction is:
rate = k[H2(g)][NO(g)]2
A three-step mechanism has been proposed for this reaction:
Step 1: 2NO(g) → N2O2(g)
Step 2: N2O2(g) + H2(g) → N2O(g) + H2O(g)
Step 3: N2O(g) + H2(g) → N2(g) + H2O(g)
i. Explain why the second step must be the rate determining step.
ii. Deduce the rate equation that would have been obtained if:
(i) the first step was the rate determining step.
(ii) the third step was the rate determining step.
Carbon monoxide can be oxidized by nitrogen(IV) oxide to form carbon dioxide. The nitrogen(IV) oxide is reduced to nitrogen(II) oxide:
CO(g) + NO2(g) → CO2(g) + NO(g)
The experimentally determined rate equation for this reaction is rate = k[NO2(g)]2.
Suggest a mechanism for this reaction which is consistent with the above information.
A two-step mechanism has been proposed for the reaction between chlorine and nitrogen(II) oxide:
Step 1. NO(g) + Cl2(g) → NOCl2(g) (fast)
Step 2. NOCl2(g) + NO(g) → 2NOCl(g) (slow)
i. State the overall balanced equation for this reaction.
ii. Deduce the order of the reaction with respect to chlorine gas and the overall order of the reaction.
iii. Deduce the rate equation for this reaction.
A teacher asked for help in solving the following non-IB question. She wrote “ Could someone explain to me how they would solve this question? I’m just confused because normally, shouldn't one concentration be held constant while the other is changed?
The following data was obtained for a particular reaction at a constant temperature:
A(g) + 2B(g) → products

Deduce the value of z in this reaction
A question with data in this form has never been asked by the IB, but by answering the following questions you can learn much about rate expressions and reaction mechanisms.
i. Explain whether the value for the rate constant can be obtained using only data given in Experiment 1.
ii. The value of z given in the mark scheme is 8.00, i.e. the rate for Experiment 4 is 8.00 mol dm–3 s–1
Show how the experimental data provided lead to this value.
iii. Calculate the value for the rate constant that is consistent with this value of z.
iv. Propose a mechanism that is consistent with this value for z.
v. Identify another value for z which is consistent with the experimental data given.
vi. Calculate the value for the rate constant if this different value for z is correct.
vii. Propose a mechanism that is consistent with this different value for z.
viii. Comment on the two mechanisms you have proposed.

 IB Docs (2) Team
IB Docs (2) Team