20.1(4) Reduction reactions
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of reduction reactions, the fourth part of the syllabus content of Topic 20.1 Types of organic reactions. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)
 Learning outcomes
Learning outcomes
.png) After studying this sub-topic you should be able to:
After studying this sub-topic you should be able to:
Understand:
- Aldehydes can be reduced to primary alcohols.
- Carboxylic acids can be reduced to primary alcohols.
- Ketones can be reduced to secondary alcohols.
- Typical reducing agents are sodium borohydride, NaBH4, and lithium aluminium hydride, LiAlH4, (used to reduce carboxylic acids).
Apply your knowledge to:
- Write equations for the reduction reactions of aldehydes to primary alcohols, ketones to secondary alcohols and carboxylic acids to primary alcohols, using suitable reducing agents.
- Describe the two-stage conversion of nitrobenzene to phenylamine.
Relationships & vocabulary
Nature of science
Organic chemical reactions involving functional group interconversions are among the key factors responsible for the progress made in the development and applications of scientific research.
International-mindedness
The misuse of alcohol is a serious problem in many countries and can have a detrimental impact both on their economies and on their social structures.
For more examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 10 & 20 : Organic chemistry.
Vocabulary
| sodium borohydride, NaBH4 | lithium aluminium hydride, LiAlH4 | phenylamine, C6H5NH2 |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Both sodium borohydride and lithium aluminium hydride are white inorganic solids but you are not very likely to have 'hands on' experience of either of them in a school laboratory. Sodium borohydride can be used in the presence of a protic solvent such as water or ethanol so is relatively safe to use but if you do use lithium aluminium hydride practically in your school then it is wise to be alert to the potential risks associated with it. It can react violently with water and even reacts with the water vapour in the air.
LiAlH4(s) + 4H2O(l) → LiOH(aq) + Al(OH)3(s) + 4H2(g)

 In 2011 it was reported that scraping an old sample of lithium aluminium hydride with a spatula caused a flash fire in a university laboratory (see above) – probably because it reacted with moisture in the air to form hydrogen gas. This potential hazard that friction can cause a fire is listed under the properties of lithium aluminium hydride on PubChem.
In 2011 it was reported that scraping an old sample of lithium aluminium hydride with a spatula caused a flash fire in a university laboratory (see above) – probably because it reacted with moisture in the air to form hydrogen gas. This potential hazard that friction can cause a fire is listed under the properties of lithium aluminium hydride on PubChem.
Considering that the new programme is so keen on using modern terminology as stated in the IUPAC Gold Book elsewhere on the syllabus (e.g. carboxamide rather than amide, phenylamine rather than aniline etc.) it seems slightly strange that the IB still refers to two of the most important reducing agents used in organic chemistry, sodium borohydride and lithium aluminium hydride, by their old names. The preferred IUPAC names for sodium borohydride, NaBH4, and lithium aluminium hydride, LiAlH4, are sodium tetrahydridoborate(III) and lithium tetrahydridoaluminate(III) respectively.
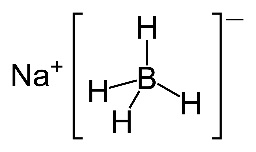
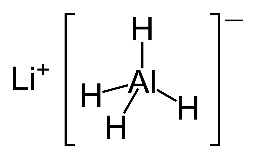
Both can be considered to be a source of hydrogen anions, H−, and they reduce carbonyl compounds by quite complex nucleophilic addition reactions.
Generally lithium aluminium hydride is a more powerful reducing agent than sodium borohydride so is preferentially used to reduce carboxylic acids to primary alcohols via the aldehyde intermediate whereas either can be used to reduce aldehydes or ketones to their respective alcohols. When carrying out these reduction processes with lithium aluminium hydide it is important to keep water out of the first step and ethoxyethane (diethylether), C2H5OC2H5, is often used as an aprotic solvent. This is dried beforehand using sodium metal which reacts with any water present and is then removed before the solvent is required. Once the intermediate has formed, acid, or sometimes an alcohol, is added to obtain the product.
One question you might ask is why the reduction of nitrobenzene is included in this sub-topic. This appears to be an isolated reaction which does not easily relate to any other chemistry on the syllabus except for the fact that the formation of nitrobenzene is given as an example of electrophilic substitution. The reaction is important as the product, aniline (and its analogues), is the precursor for the aniline dye industry (see Mauve: How One Man Invented a Colour That Changed the World in Books for enjoyment ). Aniline is also used in the production of polyurethanes and paracetamol is synthesised by reacting 4-aminophenol with ethanoic anhydride. However, as none of the reactions of aniline appears anywhere in the core. AHL or any of the options, the inclusion of the reduction of nitrobenzene seems to be just demanding the recall of factual information with no real purpose. I have given tin and concentrated hydrochloric acid as the reducing agent as it can be carried out practically in a school laboratory. It also fulfils the syllabus requirement of a two stage conversion but in fact nitrobenzene can also be reduced directly to phenylamine using hydrogen gas and a palladium or nickel catalyst at 200-300 oC.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Reduction reactions.
For short-answer questions see Reduction reactions.
More resources
1. An interesting video which goes beyond the syllabus requirements to explain why lithium aluminium hydride is a stronger reducing agent than sodium borohydride. However you should be able to follow much of it as it is based upon the relative electronegativities of boron and aluminium.
![]() Why LiAlH4 is a stronger reducing agent than NaBH4
Why LiAlH4 is a stronger reducing agent than NaBH4
2. A video showing practically how nitrobenzene can be converted into phenylamine. The reducing agent used is iron rather than tin but the principle is similar.
![]() Reduction of nitrobenzene to phenylamine
Reduction of nitrobenzene to phenylamine

 IB Docs (2) Team
IB Docs (2) Team 










