Enthalpy changes
Introduction
This is an excellent practical and well worth doing for several different reasons. It is divided into two parts – in a sense two separate experiments. The first determines directly the enthalpy change for the redox reaction between zinc metal and Cu2+(aq) ions. Because the reaction is not instantaneous students are introduced to the idea of extrapolation of a graph to determine what the temperature rise would have been if no heat had been lost to the surroundings. It is also a good experiment to introduce them to the use of a data logger with a temperature probe. The techniques learned in the first experiment can then be applied in the second experiment which involves the indirect determination of the enthalpy change for the hydration of anhydrous copper(II) sulfate using Hess’s Law. Either of the experiments could be used to cover the mandatory laboratory component listed under Topic 5.1. "A calorimetric experiment for an enthalpy of reaction should be covered and the results evaluated". Since this is a redox reaction it also covers the Higher Level mandatory laboratory component listed in Topic 19.1. "Perform lab experiments which could include single replacement reactions in aqueous solutions". The experiments could also form part of the Scaffolding required in preparation for a student who wishes to carry out his or her Individual Scientific Investigation in an area involving calorimetry.
Teacher’s notes
It is important that students follow the instructions carefully. It is necessary to take the temperature of the copper(II) sulfate solution for two minutes before adding the zinc as they need to know that the solution it is at room temperature before starting the reaction. They also need to continue taking temperature readings even after the reaction appears to have finished as they need the temperature to fall by about 2 degrees so that the graph can be extrapolated (see below).
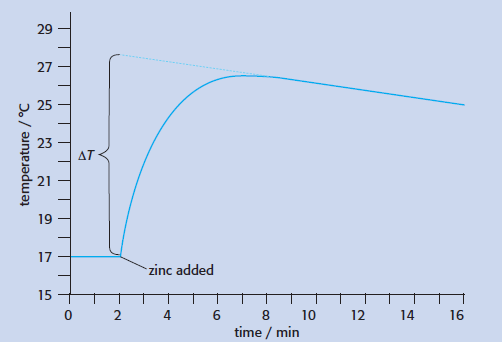
Check that they only extrapolate back to when the zinc was added and not right back to when the time equals zero. Qualitative observations are important too. Good students will observe that a gas is evolved. This is hydrogen as copper sulfate solution is acidic due to salt hydrolysis and the zinc reacts to give hydrogen as well as copper metal – this is not obvious from the equation and should be commented on in the evaluation. The literature value for this reaction (taken from The Handbook of Chemistry and Physics edited by John R Rumble) can be found to be -218 kJ mol-1 and students should obtain a value close to this. One of the major assumptions is that the specific heat capacity of the solution is the same as that for pure water and that the density of the solution is 1 g cm-3 as a volume rather than a mass is taken. Students can learn much from this.
They can then put all they have learned into practice on the second experiment where they have to do two separate enthalpy experiments – one exothermic and one endothermic - and construct their own energy cycle to obtain the value they need indirectly. For the qualitative observation students should record that the ‘white’ anhydrous copper(II) sulfate is in fact pale blue as it will have already absorbed some water from the atmosphere (unless you used a brand new sample). This is good for when they come to evaluate the practical as they should ensure the sample is dehydrated fully before commencing the experiment along with all the other obvious improvements. The literature value for the hydration enthalpy of copper(II) sulfate is -78 kJ mol-1. You can either give this value directly to the students or ask them to work it out themselves from: ∆Hf⦵(CuSO4)= – 770 kJ mol-1, ∆Hf⦵(CuSO4 .5H2O) = – 2278 kJ mol-1 and ∆Hf⦵(H2O(l)) = – 286 kJ mol-1.

 Student worksheet
Student worksheet
ENTHALPY CHANGES
In the first experiment ΔH for the reaction of zinc metal with copper(II) ions is determined directly and account is taken of heat losses by extrapolation of an appropriate graph. The second experiment involves the indirect determination of an enthalpy change using Hess's Law. Although the temperature changes can be measured mIB Docs (2) Teamally with a thermometer this experiment is ideal for using a data logger with a temperature probe.
ENVIRONMENTAL CARE:
The first experiment involves both copper and zinc and the residue should be placed in the container marked 'Heavy Metal Waste'. In the second experiment you will end up with two pure solutions of copper(II) sulfate. These should be placed in the marked bottle in the fume cupboard as they can be used for electrolysis experiments later.
SAFETY:
There are no particular hazards associated with these two experiments, although both Cu2+(aq) and Zn2+(aq) ions are poisonous so wash your hands thoroughly afterwards.
PROCEDURE:
1. Direct determination of Δ H for the reaction Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
Using a measuring cylinder, p ut 50 cm3 of 0.20 mol dm-3 copper(II) sulfate solution into a polystyrene cup and record the temperature every half minute. After two minutes add 1.2 g of zinc powder and stir the mixture thoroughly and continuously, recording the temperature every thirty seconds. From the graph plotted record the predicted temperature rise if there had been no heat loss. Take the specific heat capacity of the solution as being equal to that of water, i.e. 4200 J kg-1 K-1, and ignore the heat capacity of the calorimeter, stirrer and thermometer/data logging probe to calculate the value for ΔH.
2. Indirect determination of ΔH for the hydration of anhydrous copper(II) sulfate.
If anhydrous copper(II) sulfate powder is left in the atmosphere it slowly absorbs water vapour giving the hydrated solid.
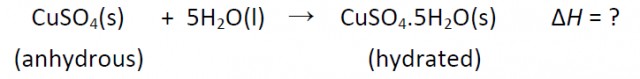
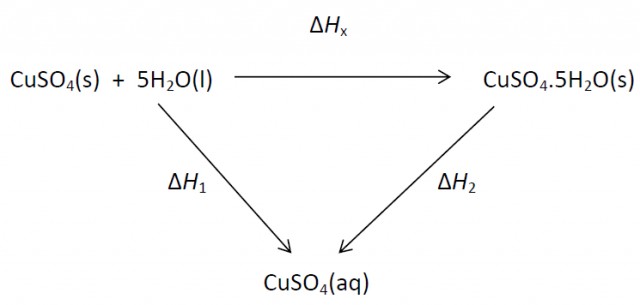
Heat is evolved in the reaction, but it is difficult to measure by the direct method. However it is possible to measure the heat changes directly when both anhydrous and hydrated copper(II) sulfate are separately dissolved in water and then construct an energy cycle to determine the required ΔH value indirectly.
1. Determine the enthalpy change when 0.025 mol of anhydrous copper(II) sulfate is dissolved in 50 cm3 of water using the same method as before.
2. Determine the enthalpy change when 0.025 mol of hydrated copper(II) sulfate is dissolved in 50 cm3 of water. (Note that 50 g water less 5 x 0.025 mole of water which comes from the hydrated salt should be weighed out).
3. Use the values you obtain from 1. and 2. to calculate the value of ∆Hx for the hydration of CuSO4.
4. Evaluate your experiment fully.
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team