Topics 4 & 14
Including International-mindedness, TOK, and 'Utilization' in Chemical bonding & structure
International-mindedness
![]()
![]() 1. Representation of Lewis structures
1. Representation of Lewis structures
Some years ago I was moderating assistant examiners as part of my role as Principal Examiner for Paper 2. I was surprised to find that an examiner based in the UK was marking correct Lewis structures wrong. The reason was not difficult to see. The answers had been written by a student from the European mainland where the accepted convention for depicting a pair of electrons is to use a line. In the UK a line is only used to show a shared (bonding) pair of electrons, otherwise electrons are shown using dots and/or crosses. In fact on British A Level papers students are not asked not to draw Lewis structures but asked instead to draw ‘dot and cross’ diagrams. An examiner who had only worked in the UK may well not have seen the use of lines before. This is one of the reasons why potential examiners are now trained before they commence marking for the IB. We often think of International -Mindedness as being the same across international boundaries but it is also interesting to look at the differences. I stand to be corrected but in the USA I believe it is common to use just dots or just crosses rather than both to represent electrons and that lines are not so common? If you look carefully at the syllabus under 4.3 Covalent structures (1) you will see that all the representations are acceptable in the IB ("Electron pairs in a Lewis (electron dot) structure can be shown as dots, crosses, a dash or any combination").
![]()
![]() 2. The ‘octet rule’ versus ‘expanding the octet’
2. The ‘octet rule’ versus ‘expanding the octet’
Ask your students to draw the Lewis structure of sulfur dioxide. The facts are that the molecule has a ‘bent’ shape and that both of the S―O bonds are of equal length and the length lies somewhere between a single S―O bond and a double S―O bond.
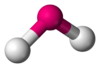
A model showing the bent shape of sulfur dioxide and equal sulfur to oxygen bond lengths
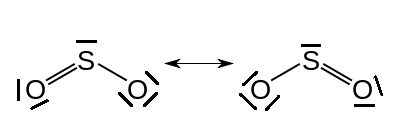
What Lewis structure(s) will you mark correct? Again I may be making a huge generalization but I think that many teachers in the USA would tend to go for the resonance hybrid model where the ‘octet rule’ can be maintained. There must be more than one structure if this model is used otherwise one oxygen atom would have a double bond and the other a single bond to the sulfur.
Many teachers in the UK would expect their students to expand the octet so that there are ten electrons around the central sulfur atom and both of the sulfur to oxygen atoms are double bonds (which presumably have a longer length than in most other compounds containing sulfur to oxygen double bonds).
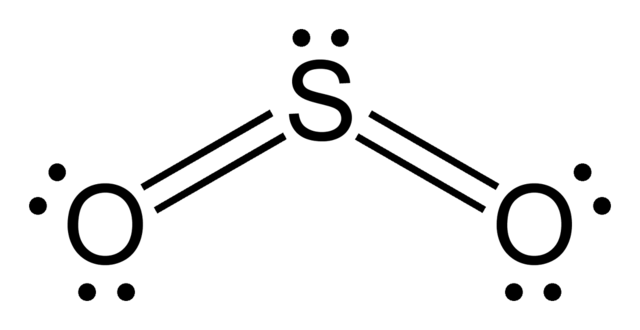
It is interesting to note that both models will enable the correct shape to be predicted using VSEPR theory[1]. Supporters of the octet rule may ask why it is necessary to expand the octet and a similar argument can also be used for sulfur trioxide. The repost is that for sulfur hexafluoride the octet has to be expanded so if it can be expanded for SF6 why not for SO2 and SO3 as there are available d orbitals on the sulfur that can be employed. This is another example of how chemistry is interpreted differently in different countries.
Theory of Knowledge
Many of the topics covered under TOK in the various sub-topics of Bonding and Chemical structure are really very similar to the content given in the Nature of Science sections. For example, what evidence do we have for theories, how reliable are they and how many exceptions have to exist before a rule ceases to be useful. This is very similar to how a paradigm gradually gets twisted to accommodate new facts which do not fit it until eventually there is a paradigm shift. Other questions asked include "To what extent is having alternative ways of describing the same phenomena a strength or a weakness?" and "Is hybridization is a real process or a mathematical device?" It is also worth including the role of dreams (i.e. less rational ways of knowing) in making scientific breakthroughs, such as Kekulé’s proposal for the cyclic structure of benzene. Essentially the examples given show that there really is no difference between TOK and NOS as I've pointed out before.
Some of the many fertile areas to include TOK/NOS discussions under the topic of Bonding and chemical structure include:
![]()
![]() 1. Representations and validity of molecular models
1. Representations and validity of molecular models
Sub-topic 4.3 looks at the shapes and bond angles for molecules and simple ions having 2, 3 and 4 electron domains (note the change from pairs of electrons) around the central atom. We can represent molecules by building models to demonstrate these shapes. These models can aid our understanding of how molecules behave but they can also hinder our understanding as they have many limitations. There is more about the use of models in Chemistry in the NOS page on key terms and concepts.
![]()
![]() 2. The nature of forces of attraction
2. The nature of forces of attraction
It is also worth questioning the very nature of the topic itself. Exactly what is a chemical bond? The syllabus (4.2.) states that a covalent bond is the electrostatic attraction between a pair of electrons and positively charged nuclei. Fine, but what about the attraction between two non-polar atoms or molecules? Perhaps the simplest example of all is helium. A helium atom is completely electrically neutral and has very small mass (in grams it is approximately equal to 4 divided by Avogadro’s constant which makes it about 6.6 x 10-24 g, or 6.6 x 10-27 kg). There clearly must be some attraction between helium atoms as it condenses to a liquid at 4 K (– 269 oC) (see the video from Nottingham University below).
Helium ![]() from Nottingham University Periodic Table Series
from Nottingham University Periodic Table Series
All bodies with mass (however small) will create a gravitational force but this can be calculated to be much too small to account for the fact that helium molecules form intermolecular forces strong enough to liquefy it at 4 K. The usual answer given is that the helium molecules form 'temporary dipoles' as they approach each other and that these are responsible for the intermolecular forces of attraction that clearly exist. This argument is then extended for heavier non-polar molecules containing more electrons. It is often said that the strength of London dispersion forces (the term used to describe these weak intermolecular attractions) depend on the number of electrons present in the molecule. However if we find examples of molecules with the same number of electrons but different molar masses we can see that their boiling points differ so it cannot depend simply on the number of electrons.
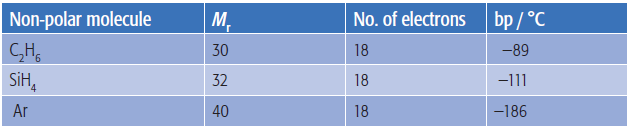
Similarly if we take molecules with the same molar mass but a different number of electrons they too have different boiling points.
.png)
No-one yet has really come up with a completely satisfactory explanation as to why non-polar molecules attract each other, although the shape of the molecule obviously does have an important role as it affects the surface contact area between the molecules. For example, if you compare the two isomers pentan-1-ol, CH3CH2CH2CH2CH2OH (b.pt: 137 oC) and 2,2-dimethylpropan-1-ol, C(CH3)3CH2OH (b.pt: 114 oC) they both have the same molar mass and number of electrons but their boiling points depend upon how spherical the molecules are. The more linear the shape the higher the boiling point as there is greater surface area contact between the molecules.
![]()
![]() 3. A modern paradigm
3. A modern paradigm
The way in which our understanding of chemical bonding has changed over time provides an excellent example of how a paradigm at first seems to work, then has to be adapted so that new information can still be accommodated until eventually information does not fit and a paradigm shift occurs to produce a new model which encompasses all of the old and also explains the new. A full description of this example can be found in the main TOK section under A modern paradigm.
![]()
![]() 4. Linus Pauling and the ad hominen fallacy
4. Linus Pauling and the ad hominen fallacy
 One of the best known names associated with chemical bonding is Linus Pauling (1901-1994). Pauling wrote many articles and books including 'The nature of the chemical bond' and was awarded the Nobel Prize for Chemistry in 1954 and the Nobel Prize for Peace in 1962. Because he was the recipient of two Nobel Prizes in different areas he was extremely respected. Late in his life he advocated that taking vitamin C would reduce the likelihood of catching the common cold. When studying logic in TOK classes students are introduced to different types of fallacies - i.e. the use of illogical arguments to 'prove' a point. One of these is known as the ad hominen fallacy. This follows the 'logic' that the argument or proposition must be true because the person saying it is such an authority on the subject. Pauling's vitamin C provides chemistry students with a good example of this because many people even now still believe it, despite the fact that there is no scientific evidence to support it.
One of the best known names associated with chemical bonding is Linus Pauling (1901-1994). Pauling wrote many articles and books including 'The nature of the chemical bond' and was awarded the Nobel Prize for Chemistry in 1954 and the Nobel Prize for Peace in 1962. Because he was the recipient of two Nobel Prizes in different areas he was extremely respected. Late in his life he advocated that taking vitamin C would reduce the likelihood of catching the common cold. When studying logic in TOK classes students are introduced to different types of fallacies - i.e. the use of illogical arguments to 'prove' a point. One of these is known as the ad hominen fallacy. This follows the 'logic' that the argument or proposition must be true because the person saying it is such an authority on the subject. Pauling's vitamin C provides chemistry students with a good example of this because many people even now still believe it, despite the fact that there is no scientific evidence to support it.
![]()
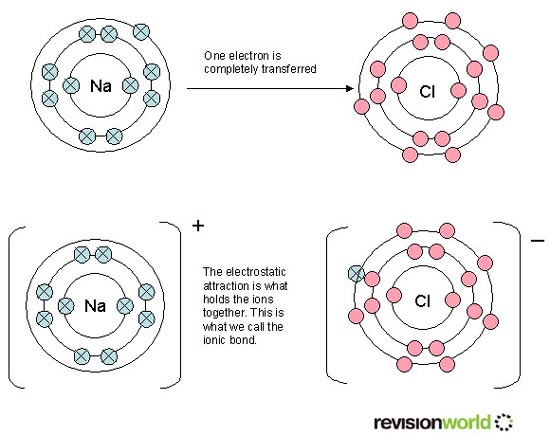
![]() 5. How ionic bonding is explained in text books or on websites
5. How ionic bonding is explained in text books or on websites
See if you can find any other criticisms apart from those listed on the page on Ionic bonding about the following typical explanation for ionic bonding (taken from revisionworld) and then look at the page I have written on 4.1 ionic bonding and structure together with the accompanying data response question which gets students to think critically about this.
'Utilization'
![]()
![]() 1. Greenhouse gases
1. Greenhouse gases
When covering carbon dioxide both in terms of its bonding (4.2.) and linear structure ((4.3) it is worth mentioning that bonds vibrate. Vibration is due to bonds stretching and bending. Some of these vibrations cause a change in the dipole moment of the molecule. When this happens the molecule absorbs radiation in the infra-red region of the spectrum.
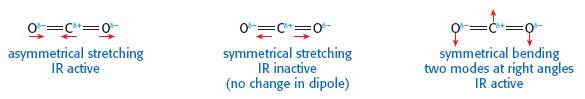
Carbon dioxide in the air allows the higher energy radiation from the sun to pass through the atmosphere but because of the asymmetric vibrations its molecules absorbs the lower energy infrared radiation (heat) from the Earth preventing it from escaping into space. This is known as the greenhouse effect and most scientists think that the warming of the Earth’s atmosphere which has occurred over the past 100 years is due to the increase in carbon dioxide in the atmosphere which is mainly caused by the burning of fossil fuels. This can lead on to a discussion on the Kyoto protocol and the term ‘carbon footprint’. (Both of these, of course, could also be used to cover International-Mindedness). This also provides a good example of cross-curricula links as infrared spectroscopy is covered in 11.3 Spectroscopic identification of organic compounds and carbon footprints and global warming are covered in Option C sub-topic C.2. Fossil fuels.
![]() 2. Ozone depletion
2. Ozone depletion
The bonding in ozone is covered in 14.1 Further aspects of covalent bonding. Because the π electrons are delocalized in ozone the resulting bond lies between a single and a double bond (it has a bond order of 1.5). It is worth stressing that this makes the bond weaker than the double bond in oxygen molecules. The stronger bond in oxygen requires high energy ultra-violet radiation to break (λ = 242 nm) whereas the weaker bond in ozone breaks at lower ultra-violet radiation (λ = 330 nm).
 In the ozone layer (about 12-50 km above the Earth’s surface) oxygen molecules absorb high energy radiation to break into oxygen radicals which then combine with more oxygen molecules to form ozone. Lower UV radiation is then absorbed by the ozone as it breaks down to reform oxygen. The net result is that ultra-violet radiation from the sun is absorbed during the steady state equilibrium between oxygen and ozone.
In the ozone layer (about 12-50 km above the Earth’s surface) oxygen molecules absorb high energy radiation to break into oxygen radicals which then combine with more oxygen molecules to form ozone. Lower UV radiation is then absorbed by the ozone as it breaks down to reform oxygen. The net result is that ultra-violet radiation from the sun is absorbed during the steady state equilibrium between oxygen and ozone.
3O2(g) ⇌ 2O3(g)
Compounds, such as CFCs which contain the carbon-chlorine bond, are normally very unreactive. However in the ozone layer the ultraviolet light provides enough energy to break the C-Cl bond to form radicals and these chlorine radicals can react with ozone to deplete its concentration. The greater amount of ultraviolet radiation now reaching the Earth has resulted in an increase in skin cancers.
Footnotes
- ^ The application of VSEPR often causes students difficulties and there is a separate page discussing how to overcome this under Areas of difficulty in the exams section.

 IB Docs (2) Team
IB Docs (2) Team