Computer modelling & simulations
Introduction
During your practical programme you need to explain the importance (and limitations) of using computer models to simulate experiments and each student should have personal experience of using computer modelling. You will need to identify on the form 4/PSOW at least one experiment where a simulation or modelling has been used during the practical scheme of work and of course the Individual Scientific Investigation can involve computer simulations. There are many sites on the web where you can download good examples although you need to be careful about the level. Some universities have very sophisticated models that are probably too complex for our students. At the opposite end of the spectrum there are many very trivial examples that might be suitable for younger students but are unlikely to stimulate IB students.
Sources for simulations
Several US universities have developed some good suitable models. Many of these sites are free to use. For example, Tom Greenbowe, previously Professor of Chemistry and Coordinator of General Chemistry at Iowa State University has produced many simulations. Tom has now moved to Oregon University and his simulations are being transferred to an existing website at Oregon University which is currently being updated (see Tom's letter explaining this). This contains many simulations and animations that can be downloaded then unzipped for use.
One of the most accessible and widely used sites is the one produced by PhET at Colorado University. This contains many virtual experiments in many chemistry topics (as well as physics and biology).
Another excellent free source of simulations is from Chemistry in Context. Once you have loaded the page simply click on the image to start the simulation. An example from one of the many simulations is shown below:
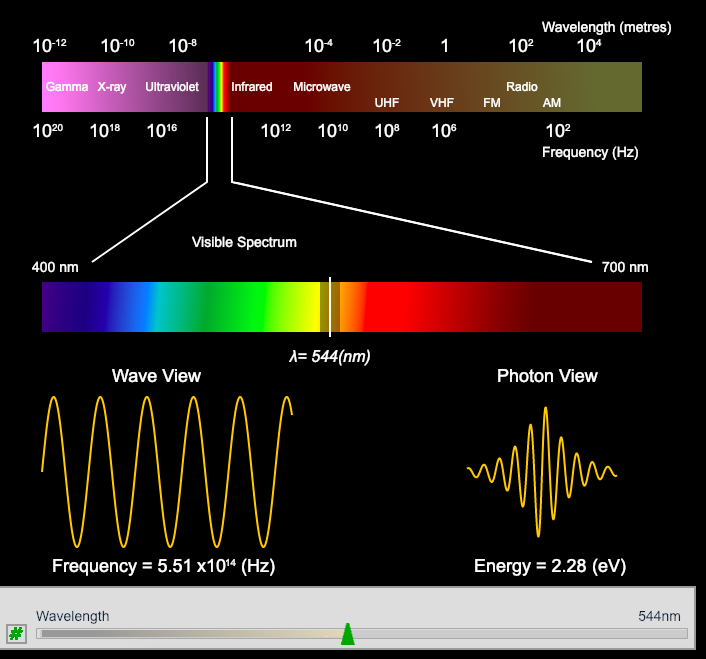
Simulation of the Electromagnetic spectrum from Chemistry in Context. Moving the slider to change the wavelength changes the frequency and energy accordingly.
There are also commercial sites that require you to pay a small fee. One such site is Model ChemLab. This originated from academic work in computer simulation and software design at McMaster University. It has continued to be developed with extensive input from educators interested in the possible application of computer simulations for classroom and distance learning.
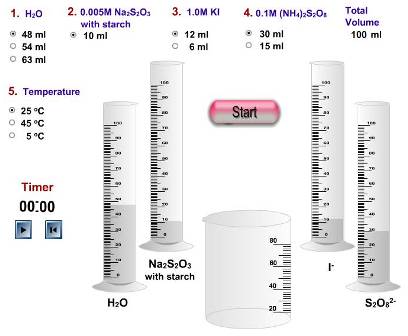
The problem with some of the simulations is that often they come a poor second to actually performing the experiment hands on. For example, one of the virtual experiments from the Iowa State University site is the iodine clock reaction (see left). By virtually altering the concentrations of the different chemicals added the time for the iodine to appear can be measured. This saves time, money and chemicals but students would have no practical experience of how to start the clock consistently when the reagents are mixed and how the temperature is kept constant throughout etc. etc.
Another relatively simple virtual experiment from Iowa State University considers electrolysis. This could be used to show the importance of the standard electrode potentials but the changes in mass illustrate Faraday's Laws which are not actually on the syllabus. Again, electrolysis is probably better as a hands on experiment - particularly to learn the skills of collecting and testing for any gases evolved.
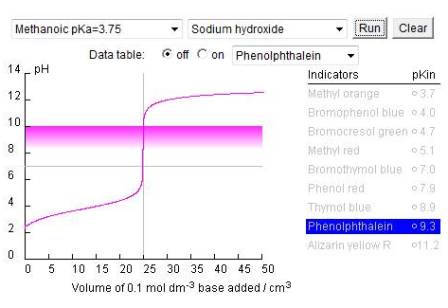
Where virtual experiments can be useful is when the students have already performed an experiment under one set of conditions then the tedium of repeating it under different sets of conditions can be avoided. A simple, but very good, example is the use of indicators in acid-base titrations. The ![]() Royal Society of Chemistry has produced a good simulation that enables the student to carry out acid base titrations using examples of both weak and strong acids and bases with a range of indicators.
Royal Society of Chemistry has produced a good simulation that enables the student to carry out acid base titrations using examples of both weak and strong acids and bases with a range of indicators.
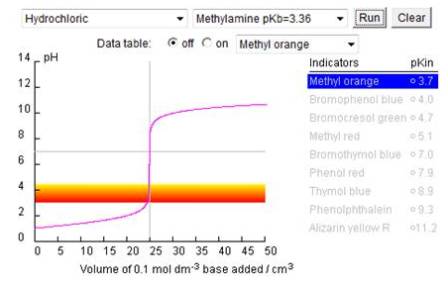
More examples of different acid-base titration curves and the effect of changing the variables have been produced by Norton college on their chemconnection pages - Norton acid-base titrations ![]() . They use ml instead of cm3 and acetic acid instead of ethanoic acid etc. but the principles are quite well illustrated.
. They use ml instead of cm3 and acetic acid instead of ethanoic acid etc. but the principles are quite well illustrated.
Other sites you may find useful are Virtual Lab at ChemCollective, KScience, Explorelearning and eduMedia. For 3-D molecular modelling you can use ACD/ChemSketch, MolView, MolCalc or Chemmybear for example.
A comprehensive list of sites offering virtual chemistry and simulations, which includes many of those I've already given above, has been put together by the American Chemical Society.
In the following two videos, James Midgley (Mr M) shows how two of the sites listed in the ACS list, ChemCollective and ChemReax can potentially be used for a student's IA. He demonstrates how to use the Virtual Laboratory in ChemCollective to make up solutions etc. and also discusses which experiments might be suitable and which are definitely not suitable for an IA.
![]() Using ChemCollective for simulation IAs
Using ChemCollective for simulation IAs
![]() Using ChemReax for simulation IAs
Using ChemReax for simulation IAs
It should be noted that if a student is using a simulation to obtain their data for their IA the IB expects them to use screen shots so that the examiner is able to get an understanding of the functionality of the simulation. These screenshots should aim to show the examiner how much input the student is able to give and to what extent the student is able to control and manipulate variables. This will allow the examiner to understand the teacher’s marking of the coursework.These screenshots should be included within the 6-12 page limit as in a sense they are replacing diagrams of hands on experimental methods.

 IB Docs (2) Team
IB Docs (2) Team