Understanding hybridization

 The theory of hybridization is developed by first considering how atomic orbitals combine to form molecular orbitals which then leads on to how hybrid orbitals are formed. The relationships between shapes, bond angles and type of hybridization are discussed together with the type of bonds (σ or π) formed and examples of the different types of questions involving hybridization that are commonly asked in examination questions are comprehensively covered.
The theory of hybridization is developed by first considering how atomic orbitals combine to form molecular orbitals which then leads on to how hybrid orbitals are formed. The relationships between shapes, bond angles and type of hybridization are discussed together with the type of bonds (σ or π) formed and examples of the different types of questions involving hybridization that are commonly asked in examination questions are comprehensively covered.
The theory
Linear combination of atomic orbitals
Understanding and applying the concept of hybridization is one of the topics that many students have difficulty with. It is only a theory and builds upon the simpler theory of the linear combination of atomic orbitals from different atoms to form covalent bonds. Possibly the difficulty with hybridization may be due to the fact that students do not really understand how molecular orbitals form from atomic orbitals as the topic is not clearly covered by the IB in sub-topic 14.1 Further aspects of covalent bonding. This is because the 'understandings' in the IB chemistry guide refers to "overlap" rather than the combination of atomic orbitals and does not include the existence of anti-bonding orbitals. To understand this consider the simplest case of the combination of two s orbitals (one from each atom) to form a sigma (σ) bond. When two s orbitals combine together they form two molecular orbitals. One of these molecular orbitals is at a lower energy level than the atomic orbitals and is known as a sigma (σ) bonding orbital. It is formed when the two s atomic orbitals combine constructively. The other, formed when the two s orbitals combine destructively, is at a higher energy and is known as a sigma antibonding orbital denoted by σ*. Overall there is no energy change as the average energy of the bonding and antibonding molecular orbitals is the same as that of the atomic orbitals.

This can be used to explain the bonding in hydrogen. Each hydrogen atom contains one electron in an s orbital. When two hydrogen atoms are brought into contact with each other the orbitals combine to give the two molecular orbitals, each of which can contain two electrons.

According to the aufbau principle both of the electrons from the two hydrogen atoms will go into the molecular orbital with the lowest energy, i.e. the bonding orbital, and a sigma bond is formed as it is more energetically favourable than if the two electrons remain in their separate atomic orbitals. Consider if two helium atoms are brought into close contact. Each contains two electrons (1s2) so two will go into the bonding orbital and two will go into the antibonding orbital so that there is no net gain in energy and so no bond is formed between the helium atoms. An analogous compound to hydrogen is the helium hydride ion HHe+, which is the strongest acid known. It is formed when H (1s1) and He+ (1s1) combine.. The compound He2+ , formed by the combination of He (1s2) with He+ (Is1), contains two electrons in the bonding molecular orbital and one in the antibonding molecular orbital so overall forms a weak ‘half’ covalent bond (described as having a bond order of ½) which is longer and weaker than the bond in H2.
When an s atomic orbital on one atom combines with a p orbital on another atom a sigma bonding and a sigma antibonding molecular orbital are formed. Similarly px and px atomic orbitals combine ‘head on’ to give sigma molecular bonding and antibonding orbitals but the combinations of py and py and pz and pz both involve ‘sideways on’ combinations and pi (π) bonding and antibonding molecular orbitals (π*) are formed.
Hybridization
To understand hybridization it is best to start by considering the bonding in methane. Each of the four hydrogen atoms has the electron configuration 1s1 and the electron configuration of carbon is 1s22s22p2. Carbon contains four outer electrons, two paired together in the 2s orbital and one each in two of the three 2p orbitals. It is not easy to see how these can form four sigma bonds without the unpairing of the two 2s electrons nor why the shape of methane is tetrahedral as the p orbitals are orthogonal (at right angles) to each other. The mixing of atomic orbitals to form new hybrid orbitals with their own properties (e.g. energy and shape) is known as hybridization. When the carbon atom bonds in methane the 2s orbital and the three 2p orbitals hybridize to form four new hybrid orbitals. This is known as sp3 hybridization and the four new hybrid orbitals, which each contain one unpaired electron, point towards the corners of a tetrahedron giving a bond angle of 109.5o. The electron in each of the sp3 hybrid orbitals is able to combine with an electron in the s orbital of a hydrogen atom to fill the four bonding sigma molecular orbitals leaving the antibonding orbitals empty resulting in four equal C−H sigma bonds. This is shown in slide 1 the gallery below which can also be found on the page on the sub-topic on 14.2 Hybridization.
Slide 2 in the gallery explains why organic compounds containing three electron domains have a bond angle of 120o as now the hybridization is sp2, i.e. three hybrid orbitals are formed from the s and two of the three p orbitals. The remaining p orbital is free to form a π bond with the p orbital of the bonding atom at right angles to the plane of the sigma bonds. This occurs in alkenes and carbonyl compounds such as aldehydes and ketones. In compounds where the central carbon atom has two electron domains the bond angle will be 180o and the two hybrid orbitals are formed from one s and one p orbital to give sp hybridization. This leaves two p orbitals which can each form a π bond with the p orbitals on the bonding atom. This occurs in carbon dioxide, alkynes and nitriles (see Slide 3). It also occurs in carbon monoxide where one of the sp hybrid orbitals on both the carbon and oxygen atoms contains a non-bonding pair of electrons and the other forms a σ bond between the carbon and oxygen atoms along with two π bonds to form the triple bond.
.png)
Hybridization and bonding in carbon monoxide
(both the C and O atom are sp hybridized with the triple bond consisting of one σ and two π bonds)
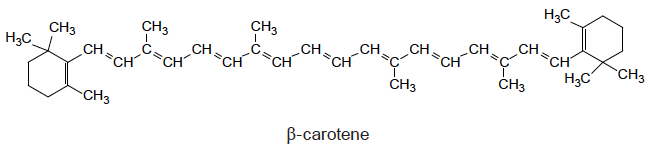
The gallery then goes on to explain the structure of benzene (Slide 5). Delocalization will occur whenever there are alternate C=C and C−C bonds as each of the carbon atoms will be sp2 hybridized so the electrons in the resulting π bonds can be spread over all the bonds equally. This results in an increase in electrical conductivity. The electron transition between the bonding and non-bonding orbitals is of lower energy in compounds containing much delocalization (also known as conjugation) than those where the bonding is localized. Hence when they are excited highly conjugated compounds tend to absorb light at a lower wavelength than compounds containing just localized bonds resulting in coloured compounds. Examples of highly conjugated molecules include porphyrins and carotenes (see for example the structure of β-carotene given below and the structures of chlorophyll, heme and α-carotene given in Section 35 of the IB data booklet).
The IB limits the types of hybridization to sp3, sp2 and sp and most examples relate to organic compounds but the concept can also be applied to other simple compounds such as NH3, NH4+ and H2O for sp3, BF3 and CO32− for sp2 and BeCl2 for sp. Other types of hybridization exist, e.g. d2sp3 for octahedral complexes, but these are beyond the IB syllabus.
Types of hybridization questions
1. Bond angles and shape
(a) Shape and bond angles from the type of hybridization
Once the type of hybridization is known the shape and hence the bond angle(s) can be predicted. For example the central carbon atom in compounds with four bonding pairs of electrons around the central atom will all be sp3 hybridized so will all have a regular tetrahedral shape with a bond angle of 109.5o. Examples include CH4, CCl4 and CHCl3. Where one of the four electron domains is a non-bonding pair of electrons the ‘parent’ shape will be tetrahedral but the actual shape will be trigonal pyramidal with bond angles slightly less than 109.5o. An example is NH3 with a bond angle of 107o. The bond angle will be even less when two of the electron domains contain non-bonding pairs of electrons to give a bent molecule, e.g. H2O with a bond angle of 105o.
sp2 hybridization leads to a trigonal planar shape with a bond angle of 120o (e.g. alkenes, ketones, aldehydes CO32−) and sp hybridization leads to a linear shape with a bond angle of 180o (e.g. alkynes, HCN).
(b) Type of hybridization from the shape or bond angle(s)
If the shape of the molecule or simple ion is known or can be deduced using VSEPR theory then the hybridization can also be deduced. If the ‘parent’ shape is tetrahedral, i.e. the bond angle is 109.5o (or slightly less) the hybridization will be sp3, if it is trigonal planar (bond angle 120o) it will be sp2 and if the shape is linear (bond angle 180o) it will be sp hybridized.
2. Types of hybridization shown in a molecule
If all the bonds around a carbon atom are single the carbon atom will be sp3 hybridized. If the carbon atom has one double bond it will be sp2 hybridized and if it has two double bonds (as in carbon dioxide) or a triple bond it will be sp hybridized.
For example:
(i) What type of hybridization is shown by each carbon atom in phenylalanine?
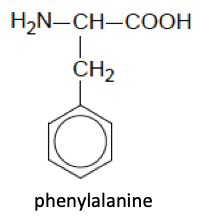

(ii) What is the hybridization of the nitrogen atom in phenylalanine?
sp3 (as there are four electron domains around the N atom - three bonding pairs and one non-bonding pair of electrons)
3. Type of bonds (σ or π ) shown in a molecule
If the carbon atom is sp2 hybridized the double bond will contain one sigma and one pi bond and if it is sp hybridized the triple bond will contain one sigma and two pi bonds. If it is sp3 hybridized all the bonds will be sigma bonds.
For example
How many sigma and how many pi bonds are present in caffeine?

24 sigma bonds and 4 pi bonds (although some delocalization occurs)

4. Change in hybridization during a reaction
When the number or type of bonds around an atom changes then there will be a change in hybridization.
The classic example is addition reactions.
For example:
.png)
5. Type of hybridization shown by allotropes of carbon
Diamond: sp3
Regular giant tetrahedral structure with four equal C−C bonds.
Graphite: sp2
Planar hexagonal rings in layers with weak bonds between the layers.
Graphene: sp2
Single layer of carbon atoms in a two-dimensional hexagonal lattice
Fullerenes: Intermediate between sp3 and sp2
Fusion of five and six membered carbon rings - see R.Hadden, Phil. Trans. R. Soc. Lond. A (1993)
Practice quiz
You should now be in a position to answer all of the ten questions which can be found in the quiz on the hybridization page.

 IB Docs (2) Team
IB Docs (2) Team 












