Polymers

 A.5 Polymers (2 hours)
A.5 Polymers (2 hours)
Pause for thought
The syllabus for this sub-topic covers the use of plasticizers to make PVC flexible but it does not actually cover what they are. Probably the most commonly used plasticizers are the phthalates which are mentioned in sub-topic A.7 Environmental impact - plastics. Technically phthalate plasticizers are dialkyl or alky-aryl esters of phthalic acid, 1,2-benzenedicarboxylic acid.
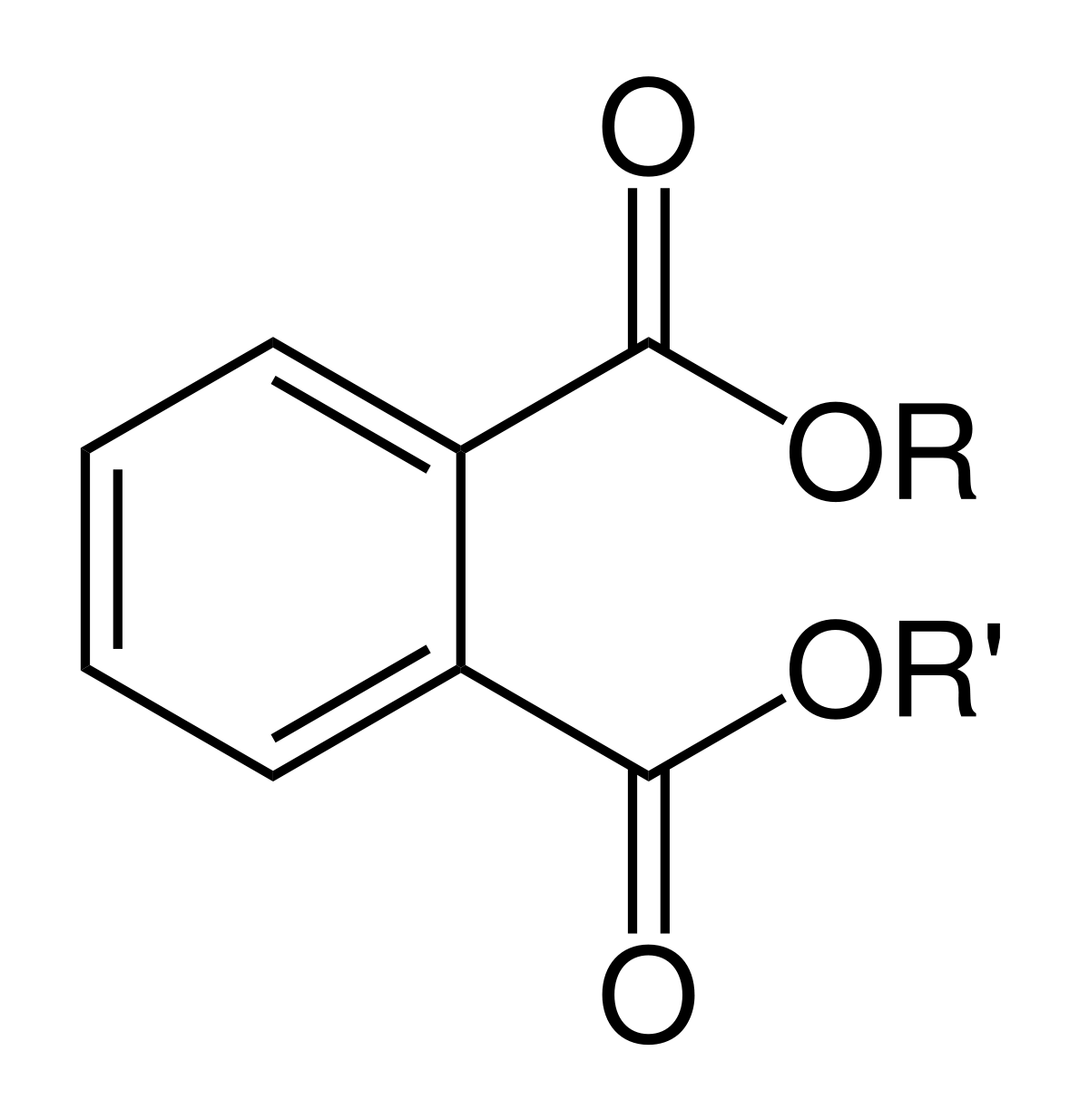
General formula for phthalate esters
They are commonly divided into two classes depending upon the molar masses of the two R- groups. Low molecular mass phthalates contain three to six carbon atoms in their R- group side chains and high molecular mass phthalates contains more than six carbon atoms in their side chains.
An example of a low molecular mass phthalate is dimethyl phthalate (DMP), R = R’ = − CH3 where Mr = 194.2, and an example of high molecular mass phthalate is di(n-octyl) phthalate (DNOP), R = R’ = −(CH2)7CH3 where Mr = 390.6.
The distinction is important because the use of low molecular mass phthalates is being phased out, particularly in North America and Europe, over concerns about safety issues. The low molecular mass phthalates are more volatile and their presence can commonly be detected in the urine of humans. Although it has been claimed that phthalates are carcinogenic there appears to be no absolute hard evidence to support this. They have also been linked to increases in asthma. In many countries their use is either completely banned or heavily restricted in certain children’s (and adults’!) toys to lower the possibility of ingestion.
Phthalates work as plasticizers in PVC by forming polar interactions between the polar δ− oxygen atoms on the C=O groups on the phthalate molecules and the δ+ carbon atoms on the vinyl chain due to the C-Cl bond. For this to happen the polymer needs to be heated in the presence of the plasticizer and the interactions then remain when the polymer is cooled thus weakening the attractions between the polymer chains and making the polymer more flexible.
Nature of science
Due to advances in technology (e.g. X-ray diffraction, scanning tunnelling electron microscopy, etc.), scientists are able to better understand polymerisation at the molecular level. This enables matter to be manipulated in new ways leading to the development of new polymers.
Staudinger's theory that macromolecules are comprised of many repeating units was integral in the development of polymer science.
The rapid development and use of polymers has increased faster than an understanding of the risks involved (e.g. recycling or possible carcinogenic properties).
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe equation for percent atom economy can be found in Section 1 of the data booklet. Examples of how the properties of polymers can be manipulated should be limited to poly(styrene) foams. International-mindednessBefore the second world war plastics were virtually unknown. Consider how the introduction of plastics has affected the world in economic, social and environmental terms. |
Teaching tipsStudents will probably have come across simple polymers before. This sub-topic is quite factual and is mainly concerned with addition polymers (although most thermosetting polymers are condensation polymers) Explain how ethene can form an addition polymer and then move on to monsubstituted alkenes so they can see the structures of poly(propene), poly(styrene) and poly(chloroethene), PVC. Stress that the properties of polymers depend upon several factors. Straight chain and branching affect the properties of poly(ethene). The less branching the more the chains can pack together (HDPE) whereas LPDE is more flexible due to increased branching and ideal for food wrapping etc. Get them to make a model of poly(propene) and they will easily see that the methyl groups on each alternate carbon atom are all either orienatated in the same direction (isotactic) or are randomly distributed (atactic). Modification includes using volatile hydrocarbons (e.g. pentane) during the polymerisation of styrene to make expanded polystyrene and the addition of plasticizers, such as phthalate esters, to PVC to make it more flexible. | Study GuidePage 116 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Polymers. For short-answer questions see Polymers questions together with the worked answers on a separate page Polymers answers. Vocabulary listthermoplastic (or thermosoftening) |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A rather nice way to see how far we have come in the past sixty years. An unashamedly propaganda film made by B.F. Goodrich in 1954 on man-made rubber.
2. A very simple explanation of how alkene monomers form addition polymers by BBLC.mov.
3. Plasticisers and Hardeners - a straightforward and clearly illustrated video by Fuseschool.

 IB Docs (2) Team
IB Docs (2) Team 















