Genuine examples of marked IA reports

This page explains in great detail through a webcast how three different genuine IAs (two databased and one hands-on) are marked and moderated. The IAs are supplied so, if you wish, you can mark them first then compare your mark with the moderators. A fourth exemplar IA is then given for you to work through to see whether you agree with the marks given.

 An IA moderator discusses how three IA reports are marked and moderated
An IA moderator discusses how three IA reports are marked and moderated
It can really help you, as a student, to see how IAs are marked as you can learn from the mistakes that other students have made and also see the standard that is required. I'm really grateful to James Midgley for sharing his experience as an IA moderator. James has supplied three IA reports which if you wish you can read through and award your own mark and then he discusses how the marks have been arrived at in this 70 minutes webcast. The first two reports are database reports, i.e. they are based on secondary data. The third is based on hands-on experimental work by the student.
![]() Explaining how three IAs are marked
Explaining how three IAs are marked
Sample IA 1 The effect of structural isomerism on the boiling points of the first six aliphatic alcohols
Sample IA 2 To What extent does the size of the central atom in H-X-H affect molecular geometry by using ligand close-packing model framework?
Sample IA 3 Determining the activation energy of the traffic light reaction.

 Do you agree with the teacher or with the IB's moderated mark?
Do you agree with the teacher or with the IB's moderated mark?
Now that you can see how IA reports are marked and moderated you might like to try this exercise.
A teacher contacted me as he was concerned that the IA report of one of his students, which he had awarded 21/24 for, was marked down to 14/24 when it was moderated by the IB.
Clearly he was perplexed as to why his marks for his students' reports had been reduced considerably and in this particular case by 33%. He asked me if I would look at the student's report to give my opinion and help him to understand "why it was marked down". Normally this is not something I have the time to do but I was intrigued that there was such a difference and wondered whether I would agree with the teacher's original marks or with the IB's moderated marks - or indeed whether I would differ from both of them. I see this as important as at workshops and in discussion forums with teachers for many years I find that they naturally worry about whether the standard of their marking is both fair to their students and in line with that required by the IB.
The report was submitted for the May 2017 session. It has the title, "Impact of Ocean Acidification on Marine Life based on a Study of the Reaction Rate between Hydrochloric Acid and Bivalve Shells". With both the student's and the teacher's permission to use the report I have completely removed any reference either by name or by number to the school, the teacher and the author but otherwise the report is as it was submitted. The teacher made one small comment which was "it is just carbonated water" after the student had written on page 3 that he/she had no means of obtaining carbonic acid. Clearly the copyright remains with the student so on the downloadable version I have acknowledged this even though it has the InThinking template.

Manila clams and mussels (the shells from the two bivalves were used in the study)
Access to the report
So that you can form your own opinion and test your own marking the IA report is reproduced below either for viewing on the site or for downloading.
My own marks and the rationale for awarding them and how they compare to both the teacher's marks and the IB's moderated mark can be seen by clicking on the 'hidden' button.
Comments and marks on the IA report:
"Impact of Ocean Acidification on Marine Life based on a Study of the Reaction Rate between Hydrochloric Acid and Bivalve Shells"
The teacher awarded the following marks:
Personal engagement 2/2
Exploration 5/6
Analysis 5/6
Evaluation 5/6
Communication 4/4
Total 21/24
When marking a student’s IA report there are two points that are worth bearing in mind before attempting to award marks for each of the five assessment criteria.
The first is that the moderating examiner will base his or her marks only on the IA report and any comments that the teacher may have added. The moderator does not know the student or the school. They will not know, for example, whether the student was keen and conscientious or whether they had to be pushed all the way to produce the report by the requisite deadline etc., so when you mark the report you need to be as objective as possible.
Secondly it is worth considering that the mark out of 24 is converted by the IB into a grade of 1-7 as one of the components of the overall grade that the student will be awarded once the three external examinations are also taken into account. The grade boundaries are arrived at by considering the Grade descriptors. For the raw marks out of 24 these boundaries are:
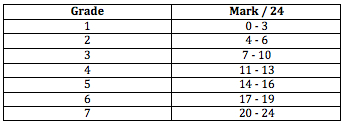
It can be very instructive to work backwards and before you address the individual criteria read the report as a whole and try to determine which grade out of 1-7 it is worthy of. When I read the accompanying IA on ocean acidification I feel that it is of Grade 5 standard, i.e. it best fits:
“Displays broad knowledge of factual information in the syllabus. Shows sound understanding of most concepts and principles and applies them in some contexts. Analyses and evaluates quantitative and/or qualitative data competently. Constructs explanations of simple phenomena. Solves most basic or familiar problems and some new or difficult quantitative and/or qualitative problems. Communicates clearly with little or no irrelevant material. Demonstrates personal skills, perseverance and responsibility in a variety of investigative activities in a fairly consistent manner. “
This means that when I sum up the marks I award for the individual internal assessment criteria I would expect the total to be in the region of 14-16. If they deviate considerably from this then it is worth re-looking at my assessment before finalising my marks.
The marks and the justification that I would give for the individual assessment criteria are as follows.
Personal engagement 1/2
This is governed by:
- Shows clear evidence of significant independent thinking or creativity.
- Demonstrates personal significance, interest or curiosity in the choice of research question or topic under investigation.
- Provides evidence of personal input and initiative in the design, implementation or presentation.
In my opinion the student has demonstrated some personal significance and interest in the choice of topic but there is not much evidence of significant independent thinking or personal input into the design of the experiment. The experiment (acid on a carbonate) is rather simplistic and the method is given as a recipe with no diagram of the apparatus used or explanation as to why the particular method was chosen compared to other possible methods.
Exploration 3/6
This is governed by:
- Identifies the topic and describes a relevant and fully focused research question.
- Contains entirely appropriate and relevant background information that enhances the scientific context of the investigation.
- Uses highly appropriate methodology to address the research question that takes into account all, or nearly all, of the factors that have a significant influence on the relevance, reliability and sufficiency of the data collected.
- Shows full awareness of any significant environmental, ethical and safety issues that are relevant to the methodology used, if appropriate.
The topic is clearly identified but the research question could be better worded as it is not clear exactly what reaction is being referred to. It would be better stated as “What effect does varying the concentration and temperature have on the rate of the reactions between hydrochloric acid and clam shells and between hydrochloric acid and mussel shells.” Unfortunately the student has not fully understood the concept of pH. It is stated that the pH has dropped 0.1 unit since the industrial revolution and is predicted to fall by a further 0.5 units but the actual pH of sea-water is not given. Carbonic acid cannot produce a pH lower than 5.65. The use of 1.0 mol dm-3 hydrochloric acid gives a pH of zero and this and the pH of 2.0 mol dm-3 hydrochloric acid are both very much lower than any change in pH caused by increased amounts of carbon dioxide dissolved in sea-water. The student has realised that carbonic acid is a weak acid but by stating that “hydrochloric acid does not impose significant limitations” has not shown that the model chosen does in fact have severe limitations. There is no attempt to determine quantitatively whether the acid or the calcium carbonate in the shells is the limiting reagent in the reaction – an important factor when planning the experiment. There is also no explanation as to why a volume of 40 cm3 of carbon dioxide was used as the cut-off point.
Analysis 4/6
This is governed by:
- Includes sufficient relevant quantitative and qualitative raw data that is able to support a detailed and valid conclusion to the research question.
- Shows that the recorded data has been sufficiently processed in an appropriate and accurate manner so that a conclusion can be drawn which is fully consistent with the experimental data.
- Shows clearly the impact of the uncertainties associated with measured data on the processed results and how they affect the conclusion.
The student has three ‘trials’ for both the clam and the mussels shells but in fact these are three separate experiments that are each only performed once. Because of the ten hour limitation this will always be a problem but it is a serious limitation on the scientific value of the data obtained. The recording and processing of the data is generally good (including the qualitative data) but the time in seconds should not be recorded to two decimal places when the it is clear from the recorded observations that this was impossible to achieve with any accuracy. There are also problems with the correct use of units. For example the student writes on page 6, “The uncertainties for molarity and temperature of hydrochloric acid are as follows ± 0.05 mL, ± 0.01g.” This is nonsense as the units for molarity are mol dm-3 and the units for temperature are oC. The ‘rate of the reaction’ in the uncertainty calculations is arrived at by dividing the volume of 40 cm3 by time whereas it would have been better to calculate the slope of the initial rate as that is what is discussed in I, II and III on page 9.
Evaluation: 3/6
This is governed by:
- Presents a detailed conclusion that is consistent with the facts presented and is completely relevant to the research question.
- Justifies the conclusion by relevant comparison to the accepted scientific context.
- Discusses the strengths and weaknesses of the investigation and the methodology used. This should include limitations associated with the data and sources of error and show a clear understanding of the factors affecting the validity of the conclusion.
- Discusses relevant and realistic ways in which the investigation might be improved and extended.
The student has recognised some of the limitations of the experiment, e.g. the importance of uniform particle size and proper control of temperature etc. and suggested some reasonable ways in which these could be overcome of at least improved, e.g. repeating the measurements to obtain an average etc. Unfortunately, although it is stated that it would have been better to use carbonic acid, the limitations of using hydrochloric acid to address the topic of acidification of the oceans are still not recognised. Different ways of measuring the amount of carbon dioxide produced, e.g. using a gas syringe or performing the experiment on a balance to record mass loss, are also not mentioned or discussed. The student has attempted to justify why decreasing the temperature decreases the rate but in fact misses the main the point that less of the reactants will possess the necessary activation energy (a decrease in the frequency of collisions is only a minor factor as most collisions do not result in a reaction).
Communication 3/4
This is governed by:
- Is written and presented clearly so that any errors do not hinder the understanding of the focus, process and outcomes.
- Is well structured so that the necessary information relating to focus, process and outcomes is presented coherently.
- Is concise and relevant so that the focus, process and outcomes can be readily understood.
- Uses correct chemical terminology and conventions and attributes all sources in a recognised manner.
The student has communicated quite well as the report flows logically and is within the prescribed 6-12 pages. Although there is no formal bibliography, sources have been properly attributed in footnotes. Words could have been saved by presenting the experimental method in a narrative form rather than simply giving a list of materials followed by a recipe. This would release more space to cover the discussion, evaluation and conclusion. In the graphs the vertical axes should be labelled ‘volume’ of carbon dioxide to correspond to the units given as the units of ‘amount’ are mol.
In summary my total mark is 14/24. This does lie within the predicted range of 14-16 obtained upon my holistic reading of the report. It is also in complete agreement with the IB moderated mark although there is no way of knowing whether the IB mark of 14/24 was arrived at in the same way.

 IB Docs (2) Team
IB Docs (2) Team