Topics 2 & 12 : Atomic structure
Introduction to 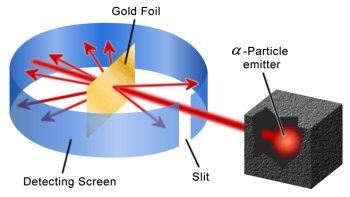

 Topic 2 and
Topic 2 and  Topic 12
Topic 12
A good understanding of atomic structure is paramount to understanding chemistry. The essential idea stated in the syllabus, that the mass of an atom is concentrated in its minute, positively charged nucleus, does not really do justice to the importance of this topic. Chemical reactions involve the rearrangement of valence electrons so understanding the structure of an atom and the nature and behaviour of electrons is paramount. This topic also brings in much Nature of Science and Theory of Knowledge as it concerns particles that are too small to ever be seen directly and provides good examples of how theories and paradigms have changed over the years. An initial glance at the syllabus is encouraging. The work of scientists like Rutherford and Thomson are referred to under ‘Nature of Science’. The ‘Aims’ include references to simulations of the gold foil experiment and use of discharge tubes.

An illustration of the Geiger and Marsden experiment to show that an atom is largely empty space.
‘Utilization’ includes reference to the use of radioactive isotopes in medicine and geological dating and reference to atomic absorption spectroscopy. It also includes PET (positron emission tomography) and its use to detect cancers. In addition CERN appears under ‘International mindedness’ and Heisenberg’s Uncertainty Principle appears under ‘Theory of Knowledge’. All of this is heady stuff and appears in the two sub-topics of the core (2.1 and 2.2) so applies to Standard Level as well as to Higher Level. According to the IB Learner Profile, you are encouraged to show curiosity and engage in critical thinking. However the amount of time given to cover this topic at Standard Level is just 6 hours. This is clearly insufficient to cover all of the above. This is recognised by the fact that what will be examined is essentially only what is listed under ‘Understandings’, Applications and skills’ (with possibly a little of what is listed under 'Nature of Science'). This means that the examinable content does not match up to material listed elsewhere. If you are a Standard Level student you will need to decide whether to just limit your study to the basic facts which can be examined or whether you wish to devote more time to understanding the underlying concepts better. You do need to know that energy levels can be split into s, p, d and f sub-levels. This is helpful when it comes to explaining several important concepts in chemistry. For example, the layout of the periodic table into blocks including lanthanoids and actinoids (3.1) and why benzene undergoes substitution rather than addition reactions (10.2). Explaining the shape of the graph obtained by plotting first ionization energies against atomic number (see image in 'Learning outcomes' above) is only required for Higher Level students (12.1). However this graph provides good evidence for sub-levels. I recommend all students try to understand it as it will help you when you are asked to deduce the electron configuration of elements and ions including the anomalies of chromium and copper and to explain the trends in ionization energies which are covered in Topic 3.2.
Note that there is no mention in syllabus of the evidence for the existence of sub-atomic particles or how their mass and charge can be measured in the examinable material, neither do you need to know or explain the operating principles of a mass spectrometer. Questions on radioisotopes cannot be asked as they are only mentioned under 'Utilization'. You will have difficulty understanding about radioisotopes and carbon dating without some knowledge of alpha, beta and gamma radiation (which is not covered by the syllabus).
Associated pages
2.1 The nuclear atom
After studying this topic you should be able to deduce the number of protons, neutrons and electrons in atoms and ions by using the nuclear symbol and perform calculations involving non-integer relative atomic masses and abundance of isotopes from given data, including mass spectra...
2.2 Electron configuration
After studying this topic you should be able to describe the relationship between colour, wavelength, frequency and energy across the electromagnetic spectrum and apply the Aufbau principle, Hund’s rule and the Pauli exclusion principle to deduce electron configurations...
12.1 Electrons in atoms
After studying this topic you should be able to use the wavelength or frequency of the convergence limit obtained from emission spectral data to calculate the value of the first ionization energy and explain the trends and discontinuities in first ionization energies across a period...
The Nature of Science
I received an e-mail from a student (not someone I teach) who asked me, "What is this nature of science business?” So what exactly does the IB mean by the Nature of Science? Essentially it covers five key points…
Topics 2 & 12
1. International scientific cooperation under the ground at CERN. Scientific research and endeavour has a long history of crossing international boundaries. 200 years ago in October 1813, while Britain...

 IB Docs (2) Team
IB Docs (2) Team