Iodination of propanone
 Background and theory
Background and theory
This practical contains a lot of good Chemistry. Students will determine a rate equation for a known chemical reaction and then use it to suggest a mechanism for the reaction. Rate equations and their relevance to reaction mechanisms are mentioned under Topic 16.1 Rate expression & reaction mechanism. Specifically the Applications and skills section states, "Evaluation of proposed reaction mechanisms to be consistent with kinetic and stoichiometric data". This practicals clearly covers this and could also be used to cover the mandatory laboratory component listed under Topic 6.1 Collision theory & rates of reaction "Investigation of rates of reaction experimentally and evaluation of the results".
A substitution reaction occurs between propanone and iodine in the presence of an acid catalyst according to the equation:
CH3COCH3 + I2 → CH2ICOCH3 + H+ + I−
The easiest way to determine the rate of this reaction is by following what happens to the colour of the iodine as it reacts to form the colourless substitution product, iodopropanone. There are several ways this can be done. One is by using a visible spectrometer (or colorimeter). If you have a data logger that can connect to a spectrometer or colorimeter then this is a good experiment to use for data logging. The video below from Malmesbury Education shows how this can be done in the laboratory. It also shows another method using titration of the amount of iodine remaining with thiosulfate solution after set periods of time.
![]() Using a colorimeter to measure the rate of iodination of propanone
Using a colorimeter to measure the rate of iodination of propanone
Another method is simply to measure the time it takes for a known quantity of iodine to disappear. The student handout uses this relatively simple method which gives students good practice at determining a rate equation by altering one concentration at a time whilst keeping all the others constant and seeing what happens to the initial rate. This is helpful as there are often questions on both Paper 1 and Paper 2 in the Higher Level examination which ask students to do exactly this.
Teacher' notes
Strictly speaking in this experiment it is not the initial rate that is being measured but it does give a close approximation. This experiment also directly tests students’ understanding of what is meant by rate of reaction and by order of reaction. When they halve the iodine concentration students will find that the time for the colour to disappear is also halved. Because the time is halved some students jump to the conclusion that the rate has doubled. They need to realise that the rate being measured is the time for a specific quantity of iodine to disappear. Hence if halving the iodine results in halving the time for the reaction then the rate is independent of the concentration of iodine and the order of reaction with respect to iodine is zero.
Proposed mechanism
Having obtained the rate equation :
Rate = k[CH3COCH3][H+]
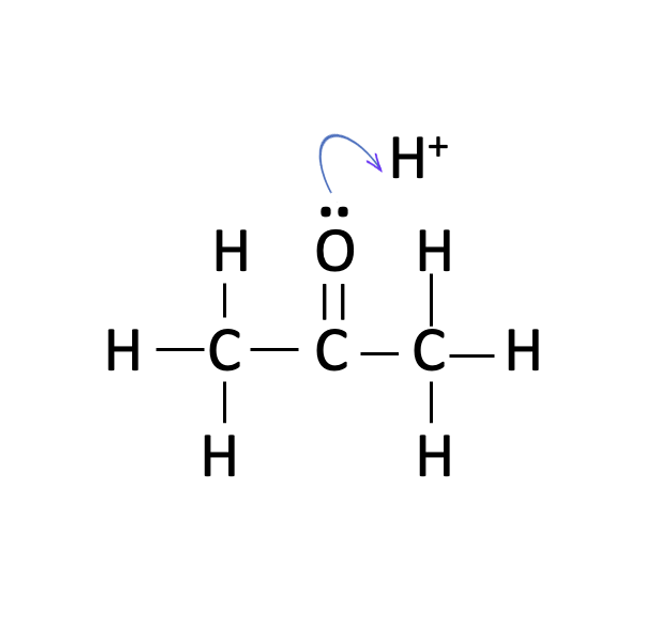
Students should be asked to propose a sensible mechanism. They should realise that only propanone and hydrogen ions take part in the rate determining step. Unless they have already covered the organic chemistry in topics 10 and 20 they may then need some help to complete the mechanism (particularly the use of ‘curly arrows’). There is actually some debate about the accepted mechanism. The first step is protonation of the oxygen atom of the carbonyl group :
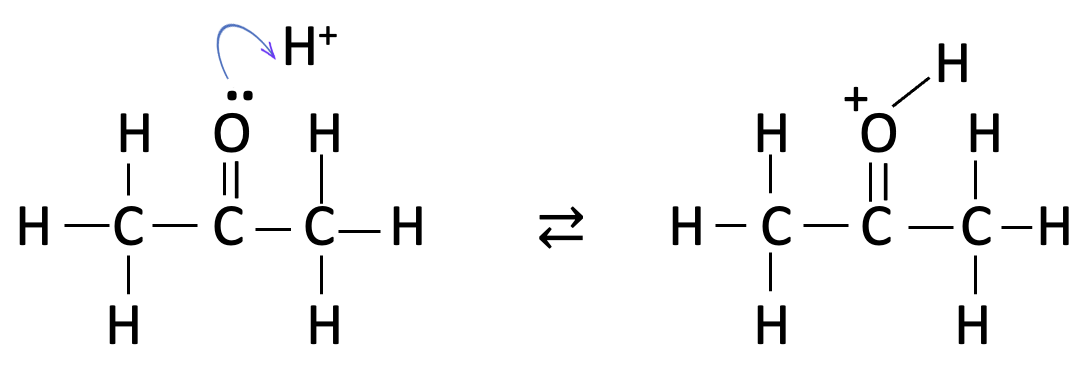
this is then followed by the formation of an enol group :
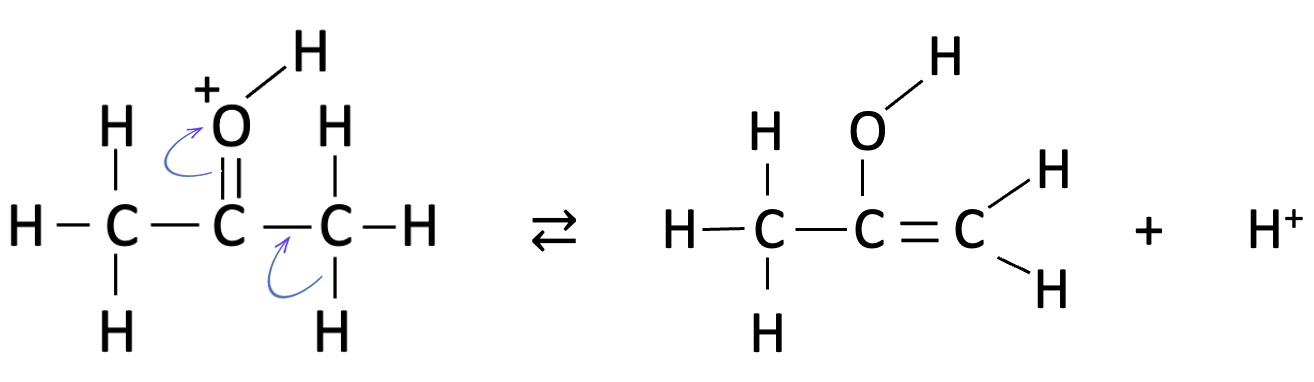
the last step is clearly the fastest as this involves addition of iodine with rearrangement back to the carbonyl group and loss of hydrogen iodide:

The problem is which one of the first two steps is the slowest - the protonation of the carbonyl oxygen atom or the rearrangement to the enol group - since both fit the rate equation as they are both first order with respect to propanone and hydrogen ions.
 Student worksheet
Student worksheet
THE KINETICS OF THE ACID-CATALYSED IODINATION OF PROPANONE
INTRODUCTION
Propanone reacts with iodine in the presence of an acid catalyst according to the equation:
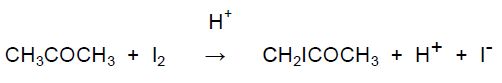
The rate of the reaction can be followed by measuring the time taken for the yellow colour of the iodine initially present to disappear. The rate equation can be expressed as:
Rate = k [CH3COCH3]x [I2]y [H+]z
(where k = rate constant and x, y and z are the orders of the reactions with respect to propanone, iodine and hydrogen ions respectively).
The aim of this practical is determine x, y and z and hence the overall order of the reaction. You will then be asked to propose a possible mechanism for the reaction which is consistent with the rate equation.
ENVIRONMENTAL CARE:
Although propanone occurs naturally, halogenated hydrocarbons can pose particular problems and even though you are only dealing with dilute solutions transfer all the residues to the marked container in the fume cupboard. Do not dispose of them down the sink. The quantities used in this experiment have been reduced from those normally recommended to limit the effect on the environment.
SAFETY:
The product, iodopropanone, is a powerful lachrymator. Even the very small vapour pressure of it above the solution can make your eyes smart, unless you are careful to keep the tubes securely corked except when you are transferring samples.
PROCEDURE:
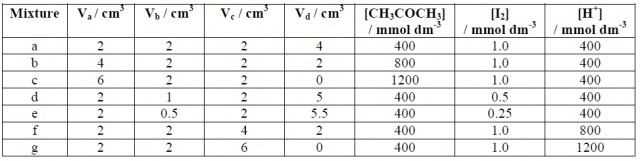
For each experiment (a - g) use a burette to put the suggested volume of iodine solution into a test-tube, and add the suggested volumes of acid and water from other burettes. Put the required amount of propanone into a separate tube. The two tubes should be added together, mixed quickly, and the stop-clock started. Note the time taken (in seconds) until the last trace of yellow colour disappears. At the end of the experiment pool your results with others in the class.
Reaction mixtures:
Va = volume of 2.0 mol dm-3 propanone(aq)
Vb = volume of 0.0050 mol dm-3 iodine (in 0.050 mol dm-3 KI)
Vc = volume of 1.0 mol dm-3 sulfuric acid
Vd = volume of water
QUESTIONS:
1. Calculate the values of x, y and z and hence give the overall rate equation.
2. Suggest a mechanism for the reaction which is consistent with the rate equation.
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team