Determining Ea for a reaction
 Background
Background

This Higher Level practical builds on one of the classic experiments normally used at a pre-IB Diploma level (e.g. GCSE) to show the factors affecting the rate of a chemical reaction. This experiment is variously known as ‘the cross on a tile’ or the disappearing cross’ reaction and involves the reaction between sodium thiosulfate and hydrochloric acid.
S2O32– (aq) + 2H+(aq) → SO2(g) + S(s) + H2O (l)
At pre-IB Diploma level the normal procedure is to look first at the effect of concentration by altering the concentration of the thiosulfate whilst keeping the concentration of the acid constant. The rate is followed by timing how long it takes for the sulfur deposit produced to cover a cross marked on a tile underneath the reaction flask. The effect of changing the temperature can also be followed by keeping the concentrations constant and warming the reaction mixture by increases of about ten degrees centigrade each time. These classic experiments can be used for a Standard Level practical to cover the mandatory laboratory component for Topic 6.1 (see Rate-dependent factors ![]() ).
).
In the UK some of the A level boards have used this experiment for planning. You could give this title as a possible source for an Individual Scientific Investigation, “An investigation into some aspect of the reaction of sodium thiosulfate with hydrochloric acid” but I would not recommend it as it is too well-known and students should be encouraged to come up with their own ideas. However, it does make a good introduction as to how an activation energy can be determined experimentally and could provide the Scaffolding for a more interesting Individual Scientific Investigation. Certainly it can be used to tick the Topic 6.1 mandatory laboratory component for Higher Level students.
I am grateful to Dr Angela Long from The Abbey School who sent me the following practical to determine the activation energy of the thiosulfate reaction. By asking students to determine the activation energy for the reaction aspects of Topic 16 are clearly being covered and students will need to understand and apply the Arrhenius equation during their data processing. Some students may need help with this so in the teachers notes below I have explained how it can be done.
Teacher's notes
Some of your students may need help to arrive at the value of Ea from their results.
The rate equation for the reaction is :
Rate = k [Na2SO3]x[HCl]y
where k is the rate constant, x is the order of the reaction with respect to sodium thiosulfate and y is the order of the reaction with respect to hydrochloric acid.
The rate is measured for the time taken for a fixed amount of sulfur to be precipitated, i.e. for a certain concentration of sodium thiosulfate to react.
Rate = Δ[Na2SO3] / t
From these two equations
k = = Δ[Na2SO3] / (t x [Na2SO3]x[HCl]y)
but Δ[Na2SO3] and [Na2SO3]x[HCl]y are constant throughout so
k = C/t where C = Δ[Na2SO3] /([Na2SO3]x[HCl]y)
From the Arrhenius equation
ln(C/t) = − Ea / RT + ln A
so ln(1/t) = − Ea / RT + ln A – ln C = − Ea / RT + ln (A/C)
A plot of ln (1/t) against 1/T (where T is the temperature in Kelvin) will give a straight line and the gradient will be equal to − Ea / R.
Alternatively ln t = Ea / RT − ln (A/C) so a plot of ln t against 1/T will give a straight line with the gradient being equal to Ea / R.
CLEAPSS give a value of about 47 kJ mol-1 for Ea for this reaction.
(Note that rather than put it into my standard format I reproduce below (and attach) Angela's own student handout as it can be interesting to see how other teachers approach practical work.)
 Student worksheet (courtesy of The Abbey School)
Student worksheet (courtesy of The Abbey School)
Research Question:
“Finding the activation energy of a reaction”
Instructions / Background:
In this experiment you will mix solutions of hydrochloric acid and sodium thiosulfate at different temperatures. A reaction takes place in which a precipitate of sulphur is formed. You will record the time from mixing to a point at which the reaction mixture is too cloudy to allow a spot to be seen through it.
The equation for the reaction is: S2O32- (aq) + 2H+(aq) → SO2(g) + S(s) + H2O (l)
You must pay attention to all aspects of safety when using these items during your experiment. You are supplied with insulating gloves to use at any time of the procedure.
Materials / Chemicals required:
- 2 boiling tubes
- 400 cm3 beakers
- Marker pen
- Stand and clamp
- Timer
- Bunsen burner, tripod and gauze
- 0 – 100 oC thermometer
- 2 x 10 cm3 measuring cylinders
- Access to a fume cupboard.
- 60 cm3 of aqueous sodium thiosulfate (approximately 0.10 mol dm-3)
- 60 cm3 of aqueous hydrochloric acid (approximately 0.10 mol dm-3)
- A4 graph paper
Safety considerations:
Do not carry out this experiment if you are asthmatic
Do NOT dispose of your reaction mixture down the sink. Pour into the supplied bucket where the reaction will be safely quenched and disposed of for you.
Do not heat the solutions above 50oC
Procedure
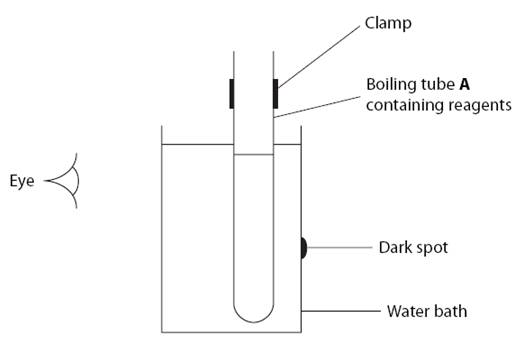
1. Label two boiling tubes A and B. Mark a dark spot on the side of a 400cm3 beaker, then half fill it with water.
Clamp tube A and immerse in the water bath as shown in the diagram above.
2. Using a measuring cylinder, transfer 10cm3 of sodium thiosulfate solution to tube A.
3. Using a clean measuring cylinder, transfer 10cm3 of hydrochloric acid to tube B and place the tube in the beaker of water.
4. Allow both solutions to reach thermal equilibrium with the water in the beaker for a few minutes.
5. Add the solution from tube B to that in tube A, starting a timer as you do so. Mix the solution in A by gently stirring using the thermometer. Read and record the temperature.
6. Observe the spot on the side of the beaker by looking at it through the solution in A. Record the time at which the spot can no longer be seen due to it becoming obscured by the sulfur precipitate formed in A.
7. Dispose of the mixture in tube A as directed. Rinse out tube and wash and dry the thermometer.
8. Using a Bunsen burner to gently heat the water bath, repeat steps 2 – 7 until you have 5 sets of results at five different temperatures. The first will be at room temperature and the other four evenly spaced between room temperature and about 50oC (try not to exceed this temperature).
9. Record your results in a suitable manner and then process your data making use of the Arrhenius equation to determine the answer to the research question.
Dr Angela Long
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team