Culture of Chemistry (3)
The Culture of Chemistry quizzes illustrate some of the Nature of Science aspects of the course. They aim to widen your general knowledge about our subject and give you some genuine examples of how chemistry works practically both now and in the past in everyday life as well as theoretically. Each quiz consists of ten multiple choice questions. Full answers and explanations are provided to hopefully increase your enjoyment and love of chemistry as well as stimulate your interest to perhaps do some further research.
Introduction
This is the third of the Culture of Chemistry quizzes. The quizzes have several aims. Essentially they illustrate some of the Nature of Science aspects of the course. They may also be helpful for your study of Theory of Knowledge as culture is one of the twelve TOK concepts. The questions are unlikely to contain content that will be examined as such in the May/November examination sessions but they aim to widen your general knowledge about our subject and give you some genuine examples of how chemistry works practically both now and in the past in everyday life as well as theoretically. They are meant to be fun and perhaps in these current Covid times also provide a little bit of light relief. I would not expect you to know many of the answers so the score you get is irrelevant but I have provided fulsome answers and explanations to hopefully increase your enjoyment and love of chemistry as well as stimulate your interest to perhaps do some further research. Some of the questions may provide you with the spark of an idea that you could develop for your individual scientific investigation (IA) or extended essay.
Culture of Chemistry (3) Quiz
Which alloy does not contain tin?
Brass is approximately two thirds copper and one third zinc. Pewter is mainly tin alloyed with antimony and small amounts of other metals such as silver, bismuth and copper. In the past it also contained lead but as pewter is often used for cups and bowls this led to lead poisoning. Solder, used to join metals together, is an alloy of lead and tin. Bronze is an alloy of copper. containing about 12% tin.
Which is correct when describing Fe in FeSO4?
.png)
Oxidation states must have + or − in front of them and use Arabic numerals. Oxidation numbers use Roman numerals in brackets with no + or −. The charge on an ion uses a Roman numeral followed by + or −. An example of the use of language in chemistry. It is worth noting that often oxidation state and oxidation number are used interchangeably.
The 2020 Nobel Prize for chemistry was awarded to Professor EmmIB Docs (2) Teamelle Charpentier and Professor Jennifer A. Doudna. They are the sixth and seventh women to be awarded the prize in chemistry and this is the first time that the Nobel Prize in any of the sciences has been awarded to two women without a male collaborator also listed on the award. What was the award for?
Charpentier and Doudna discovered the CRISPR/Cas9 genetic scissors. These can be used to change the DNA of animals, plants and microorganisms with extremely high precision. This technology revolutionised the molecular life sciences, brought new opportunities for plant breeding, is contributing to innovative cancer therapies and has the potential to the dream of curing inherited diseases come true. For more information see https://www.nobelprize.org/prizes/chemistry/2020/advanced-information/
Which statement is written correctly?
Another example of the language of chemistry. Except when starting a sentence, elements do not have capital letters. The Celsius scale of temperature is measured in degrees Celsius, ℃, and the kelvin scale is measured in kelvin, K.
Which famous chemist is being referred to in the following quotation?
"It took them only an instant to cut off this head, and one hundred years might not suffice to reproduce its like."
This quotation ""Il ne leur a fallu qu'un moment pour faire tomber cette tête, et cent années peut-être ne suffiront pas pour en reproduire une semblable." was made by the mathematician Guiseppe Lagrange after the execution by guillotine of Antoine Lavoisier during the French revolution in 1794.
What is the main symptom of 'Daltonism'?
The correct term is protanopia. It is called Daltonism after John Dalton, the father of atomic theory, who suffered from colour blindness and published a paper on it in 1794 in which he recognised that colour blindness is hereditary.
Which chemist wrote a collection of short stories called “The Periodic Table”?
Primo Levi's 21 short stories, each named after an element, are an autobiography of his life as a chemist in Italy and later in the Auschwitz concentration camp.
Which is a rare-earth element?
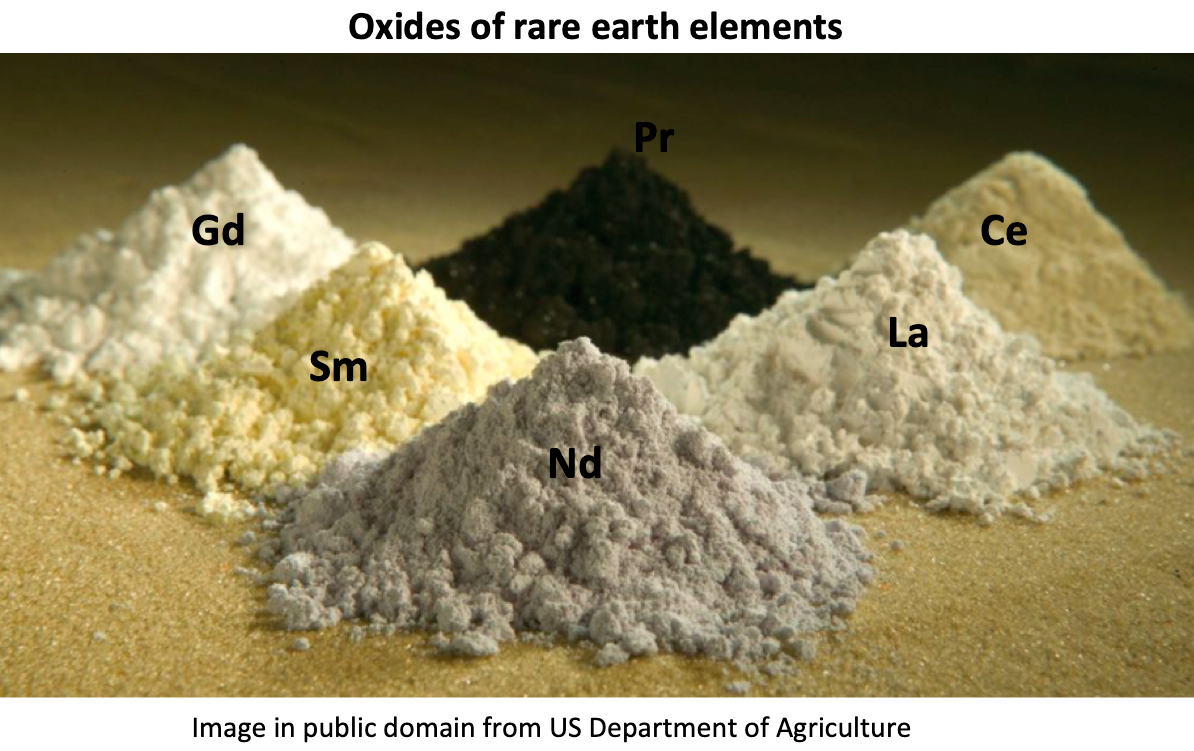
There are 17 rare-earth elements made up of the lanthanoids together with scandium and yttrium. The majority of them are not actually that rare - cerium is more abundant than copper. However they are spread thinly rather than found in concentrated ores so are less economically exploitable. They are increasingly important in electronics. For example, Nd, Ce and Ga are used in the production of LCD and plasma screens, fibre optics and lasers, other rare-earth elements are used in fuel cells and batteries for electric cars. There is concern that the world’s demands will outstrip the supply, particularly as currently China is responsible for about 80% of the world’s production.
Which novelist and scientist said the following during a lecture given in 1959?
"A good many times I have been present at gatherings of people, who by the standards of traditional culture, are thought to be highly educated and who have with considerable gusto been expressing their incredulity at the illiteracy of scientists. Once or twice I have been provoked and have asked the company how many of them could describe the second law of thermodynamics. The response was cold: it was also negative. Yet I was asking something which is the scientific equivalent of "Have you read a work of Shakespeare's?" "
C.P. Snow (1905 - 1980), a celebrated scientist and novelist, gave the Rede lectures in 1959 in which he condemned education systems that rewarded the study of the humanities at the expense of the sciences. In chemistry the most useful aspect of the second law of thermodynamics is that for a reaction to be spontaneous the value of the Gibbs energy, (ΔG), that is the energy available to do work, must be negative.
Potters use metal oxides to obtain glazes of particular colours when they fire their products in a kiln.
Which metal oxide was used to produce the red glaze in the mug shown?

Iron oxide has been used for centuries to impart a red colour to pottery. Cobalt(II) oxide is used for blue and chromium(III) oxide for green. Strontium oxide gives a red flame test when heated strongly but does not impart a colour to the glaze. It is used as a flux to make a smoother and more transparent glaze.

 IB Docs (2) Team
IB Docs (2) Team