Topics 3 & 13
3 (
 6 h) & 13 (
6 h) & 13 ( 4 h) : Periodicity
4 h) : Periodicity
Periodic tables
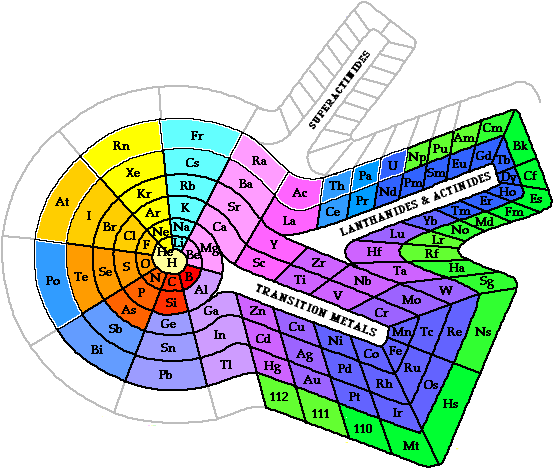 The MYP programme (unlike the Diploma programme) places considerable emphasis on the concept of human ingenuity (this is the modern (non-Latin) 'translation' of what used to be known as homo faber – or man the tool maker). It seems to me that the periodic table is almost the perfect example of human ingenuity in Chemistry. What is amazing is that more than 140 years since the periodic table was first proposed by Mendeleev in 1869 there is still not the Periodic Table. If you search in books and on the Internet you will find that there are many different periodic tables. These range from short to long forms, some with eight groups and others with eighteen groups. Periodic tables also appear in spiral (see right) and three-dimensional forms. Some have hydrogen appearing not once but twice, and its position can vary from being on its own or at the top of Group 1 or at the top of Group 7. When I was running a workshop in San Jose, the Costa Rican teachers showed me the periodic table they use in their schools (see below) which is very different to the ones that are usually accepted internationally.
The MYP programme (unlike the Diploma programme) places considerable emphasis on the concept of human ingenuity (this is the modern (non-Latin) 'translation' of what used to be known as homo faber – or man the tool maker). It seems to me that the periodic table is almost the perfect example of human ingenuity in Chemistry. What is amazing is that more than 140 years since the periodic table was first proposed by Mendeleev in 1869 there is still not the Periodic Table. If you search in books and on the Internet you will find that there are many different periodic tables. These range from short to long forms, some with eight groups and others with eighteen groups. Periodic tables also appear in spiral (see right) and three-dimensional forms. Some have hydrogen appearing not once but twice, and its position can vary from being on its own or at the top of Group 1 or at the top of Group 7. When I was running a workshop in San Jose, the Costa Rican teachers showed me the periodic table they use in their schools (see below) which is very different to the ones that are usually accepted internationally.
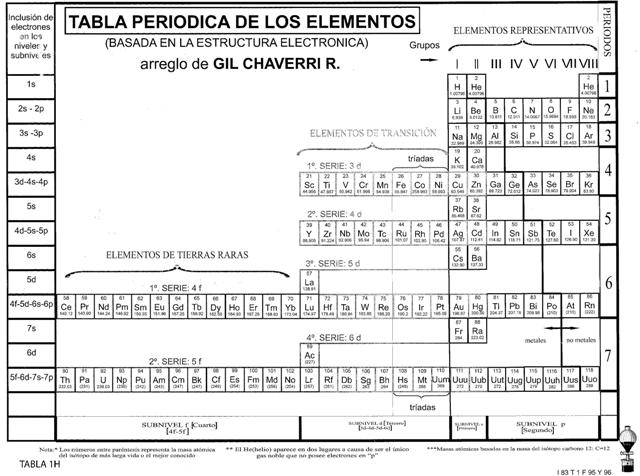
The periodic table used in schools in Costa Rica.
Artists and others have also run with the idea of the periodic table and now you can find 'spoof' periodic tables of almost anything ranging from Atheists and antitheists to Rock and Roll, typefaces and others that are too rude to mention. For teaching the IB Diploma make sure you use the version of the periodic table that is published as Section 6 the IB data booklet. I actually photocopy this and give it out separately right at the beginning of the two year course so that students get used to using it. Note that this periodic table is different from past programmes as the groups are numbered from 1 to 18.
What is 'periodicity'?
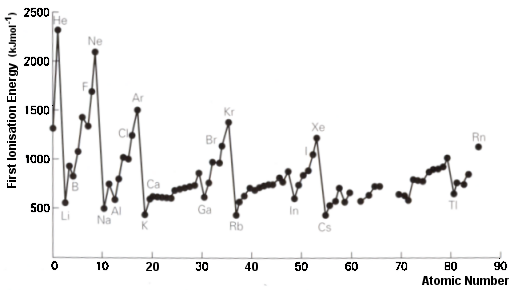
One of the strange aspects Topic 3 is that it is called ‘Periodicity’ and yet nowhere in the assessment statements are students required to understand or define the word periodicity! If you ask students what they think it means you then you often get rather vague answers. Since it is crucial to understanding the topic it is worth getting them to understand that periodicity means that when arranged according to their atomic numbers there is a repeating pattern in the chemical and physical properties of the elements. Perhaps the best example to illustrate this is the graph of first ionization energies where the distinctive repeating pattern due to the successive filling of the three p orbitals in each energy level (e.g. B - Ne, Al - Ar, Ga - Kr, and In - Xe) can be seen clearly.
Check the syllabus
When covering this topic read the 'Understandings', 'Applications and skills' and 'Guidance' carefully as the syllabus is actually quite limited in what it covers. For example, In Topic 3.2 under' Applications and skills' it states "Discussion of the similarities and differences in the properties of elements in the same group, with reference to alkali metals (group 1) and halogens (group 17)". The 'Guidance' states that group trends should include the treatment of the reactions of alkali metals with water, alkali metals with halogens and halogens with halide ions. It does not mention any other groups nor does it mention the change in the chemical properties of the elements across a period such as Na – Ar. Similarly, although the change in nature of the oxides going across period 3 is covered the equations are only required for the reaction with water to explain the pH change for just sodium(I) oxide, Na2O, magnesium oxide, MgO, phosphorus(V) oxide, P4O10 and the oxides of nitrogen and sulfur (although it does not specify exactly which oxides of sulfur and nitrogen should be covered).
Interactive resources
There are many interactive web resources that you can use to illustrate the chemistry contained in this topic. For example, ptable.com is a really good Interactive periodic table. It is set out very clearly and contains a huge amount of information on each element. Other interactive periodic tables include WebElements and Dynamic periodic table. Perhaps one of the best resources is the series of videos on each element (and more besides) produced by the University of Nottingham in their series The periodic table of videos.
The links on the left give you teaching tips etc. for each of the sub-topics together with questions and answers for each sub-topic.
For some fun 'applications' of the Periodic Table it is worth looking at Not an exact science show. The following image is taken from there.

Once you have finished teaching the whole topic you can give the multiple choice tests on Periodicity (SL) and Periodicity & Transition metals (HL) (together with answers):![]()
![]() Topic 3
Topic 3

 IB Docs (2) Team
IB Docs (2) Team