'Utilization' - Relationships between topics
'Utilization' and a holistic approach to teaching chemistry
Rationale
Because it states that it will not be examined as such, there may be a tendency to ignore much of what is listed in the 'utilization' section for each of the sub-topics. Sometimes there are links to other IB subjects or even broader references and it is probably safe to assume that these will not be specifically examined. Even so, a good teacher will explain the origin of phrases such as 'a copper-bottomed investment' (Economics) or why Haber's discovery of the fixation of nitrogen was one of the main reasons why the First World War continued for as long as it did (History). It is these asides that help to make chemistry interesting to students. However there is one aspect of utilization that definitely will be examined and that is where the reference is to another part of the chemistry syllabus. For example, in the guidance notes for sub-topic 13.2 Coloured complexes it clearly states "Syllabus and cross-curricular links: Topic 2.2—electron configuration of atoms and ions".
Making links between topics in chemistry
There is a tendency in many national systems now for teachers to teach chemistry in modules. One of the results of this is that teachers themselves lose a holistic approach to chemistry and students never gain it in the first place. I personally think that it is really good practice to relate the topic you are currently teaching to all the other ten topics on the Core/AHL part of the programme irrespective of what it states under 'utilization'. This will not only give students a much better understanding of chemistry as a whole but will also help them when they come to answer the data response question on Paper 3 and also some of the questions on Paper 2 which again often involve relationships between topics. One of the activities I have devised for use in workshops is to put the participating teachers into pairs and ask them to choose a number between 1 and 11. The number they choose then identifies which topic they put in the centre of my ‘relationships between topics’ diagram. In the following example number 4 would have been chosen so Topic 4 (and 14) - Chemical bonding & structure goes into the middle .
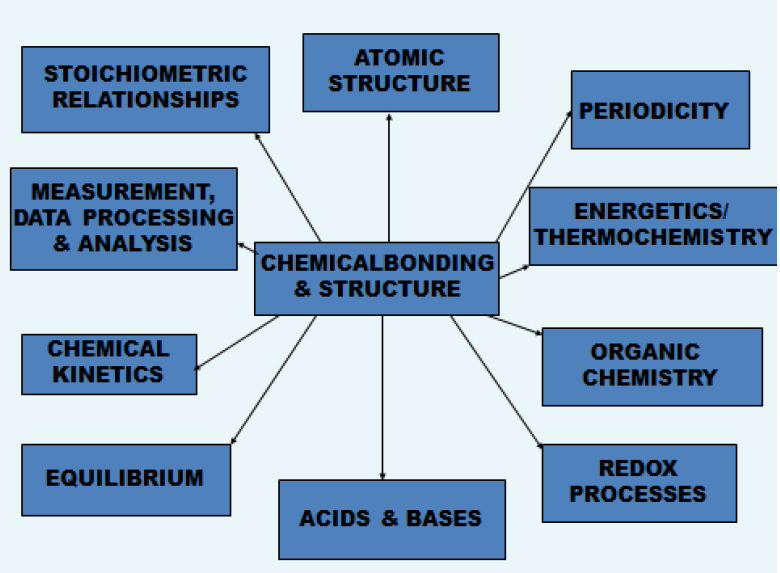
I then ask the teachers to think of two separate examples in which the central topic can be related to each of the other ten topics that they could include as they teach 'Chemical bonding and structure’.
You might like to try this for yourself. Do it several times and each time put a different topic in the middle. It is a good exercise for teachers new to the programme to get to know the syllabus and also a good exercise to give students near to the end of their course to help with their revision/review programme. Even if you are an experienced teacher I guarantee it will make you think and improve your teaching! It is perfectly possible to come up with at least two relationships between each one of the eleven topics and the other ten.
In case you do not have the time (or you are inherently lazy!) I've attached as a separate page one solution showing how Topics 4 & 14 : Chemical bonding and structure relates to each of the other topics at both Standard Level and Higher Level.
On another separate page, Example of connections, I have given an actual example of how a topic that is usually confined to organic chemistry can produce some stimulating and interesting chemistry when looked at from different perspectives.

 IB Docs (2) Team
IB Docs (2) Team