Understanding and using ΔG
What does ΔG represent and how is it used in the IB?
ΔG is the change in free energy for a chemical reaction. If it is measured under standard conditions then it is known as the standard free energy change and has the symbol ΔG⦵. If the value of ΔG⦵ is negative then the reaction is spontaneous under standard conditions whereas if the value of ΔG⦵ is positive then the reaction is non-spontaneous under standard conditions. Spontaneous means that the reaction can do work, i.e. give out energy.
The IB details three relationships for ΔG⦵
| 1. ΔG⦵ = ΔH⦵ − TΔS⦵ | where ΔH⦵ and ΔS⦵ are respectively the standard enthalpy change and the standard entropy change for the reaction at temperature T. |
| 2. ΔG⦵ = − RT lnKc | where R is the gas constant and Kc is the equilibrium constant for the reaction. |
| 3. ΔG⦵ = − nFE⦵ | where F is the Faraday constant, n is the number of electrons transferred and E⦵ is the standard electromotive force produced by the electrochemical cell. |
Problems experienced by students
The difficulties encountered by students tend to fall into one (or more) of the following three areas.
- Problems involving calculations using the equations above.
- Problems understanding how ΔG⦵ must always equal zero for a system in equilibrium.
- Problems understanding why spontaneous reactions do not always proceed spontaneously.
Let’s look at each in turn.
Problems with calculations
The most common error is not using the correct units when trying to find the value of ΔG⦵ when substituting into the equation ΔG⦵ = ΔH⦵ − TΔS⦵.
ΔG⦵ and ΔHo both have the same units. This is kJ mol−1, although for a reaction such as N2(g) + 3H2(g) ⇌ 2NH3(g) the units would just be kJ as the energy or enthalpy change is for one mole of nitrogen, three moles of hydrogen or two moles of ammonia. What causes the problem is that the entropy change, ΔS⦵ has the units J K−1 mol−1 and students forget to convert the values of ΔG⦵ and/or ΔH⦵ into J mol−1 (or convert the value of ΔS⦵ into kJ K−1 mol−1) before substituting the values into the equation. It is also important to give the value for the temperature in Kelvin (K), not degrees Celsius (oC).
Example
Nitrogen(II) oxide and oxygen react to form nitrogen(IV) oxide according to the equation:
2NO(g) + O2(g) → 2NO2(g).
Determine the value of ΔG⦵ for this reaction at 25 oC given that ΔH⦵ = − 113.1 kJ and ΔS⦵ = − 147 J K−1.
Worked solution
Convert the temperature to Kelvin and the value of ΔS⦵ into kJ K−1 mol−1 then substitute into the equation.
ΔG⦵ = ΔH⦵ − T ΔS⦵ = − 113.1 – (298 x 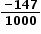 ) = − 69.3 kJ
) = − 69.3 kJ
The value of ΔG⦵ at equilibrium
Students can get confused when they use the equation ΔG⦵ = − RT lnKc for a reaction that has reached equilibrium. They may conclude that as ΔG⦵ = 0 at equilibrium then the value of Kc must always be one (since ln1 = 0) for any reaction at equilibrium, i.e. [C] x [D] = [A] x [B] for the equilibrium reaction A + B ⇌ C + D, which is clearly wrong. The problem is what is meant by ΔGo in this context?
Consider the example above. ΔGo for the forward reaction is the free energy change after starting with just stoichiometric amounts of NO(g) + O2(g) with no product present until the reaction has gone to completion to form the product.
If we had written the equation for the reverse reaction 2NO2(g) → 2NO(g) + O2(g) then the quantitative values for ΔGo, ΔH⦵ and ΔS⦵ would all be the same but their signs would all be reversed, i.e. ΔG⦵ = + 69.3 kJ, ΔH⦵ = + 113.1 kJ and ΔS⦵ = + 147 J K−1. For this reverse reaction we would be starting with just NO2(g) and the ΔG⦵ value of + 69.3 kJ represents the free energy change when this has all reacted to form 2NO(g) + O2(g). When we say that ΔG⦵ for the reaction equals zero at equilibrium we mean that we are starting with the equilibrium concentrations of reactants and products. Sometimes this is written as ΔG⦵eqm. Since at equilibrium the rate of the forward reaction = the rate of the reverse reaction, no net reaction occurs so there will be no change in free energy.
When we use the expression ΔG⦵ = − RT lnKc the value of ΔG⦵ means the value for the forward reaction as it is written to give the equilibrium expression for Kc. It is not the value of ΔG⦵ at equilibrium, ΔG⦵eqm.
Example
Determine the value of the equilibrium constant for the reaction between hydrogen and iodine at 427 oC.
I2(g) + H2(g) ⇌ 2HIg) ΔG⦵ = − 23.2 kJ
Worked solution
ΔG⦵ = − RT lnKc1
Since the data booklet value for R is 8.31 J K−1 mol−1, the value for ΔG⦵ must be converted into joules and the temperature converted from oC into Kelvin.
lnKc = − 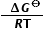 =
= 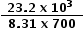 = 3.988
= 3.988
Kc = e3.988 = 53.9 at 700 K.
1For gaseous reactions Kp should really be used rather than Kc, as that is the equilibrium constant expressed in terms of partial pressures rather than concentrations, but Kp is not on the IB syllabus. For equilibrium reactions such as this where there is no change in volume Kp = Kc but this simple relationship is not true for a reaction such as N2(g) + 3H2(g) ⇌ 2NH3(g) where there is a volume change.
Why do spontaneous reactions not always proceed spontaneously?
At first glance this statement appears to contradict itself. The problem is the different meanings of the word ‘spontaneous’. Thermodynamically it means react to give out energy that can do work but its other meaning is to occur naturally with no pre-planning, i.e. ‘off the cuff’. The example above shows that as ΔG⦵ has a negative value at 700 K it will be a spontaneous reaction at this temperature and as the value of the equilibrium constant is high there will be almost all product (hydrogen iodide) and very little reactants (hydrogen and iodine) remaining in the mixture at equilibrium. However it does not give any information about the rate at which equilibrium will be reached.
.png) Just because a reaction is spontaneous it does not mean that it will actually occur in a reasonable time as the activation energy may be too high for collisions between the reacting species molecules to result in the formation of products. A good example of how the value of ΔG⦵ cannot be used to determine whether or not a reaction will actually proceed is the oxidation of coal or diamond in air to form carbon dioxide. This reaction is very spontaneous (ΔG⦵ = − 395 kJ mol−1) but coal and diamond are stable in the atmosphere at normal temperatures for millions of years. To summarise: spontaneity is a thermodynamic property and gives information about the amount of energy that can potentially be produced to do work whereas whether or not a reaction actually occurs (i.e. the rate of the reaction) is a kinetic property and essentially depends upon the activation energy of the reaction.
Just because a reaction is spontaneous it does not mean that it will actually occur in a reasonable time as the activation energy may be too high for collisions between the reacting species molecules to result in the formation of products. A good example of how the value of ΔG⦵ cannot be used to determine whether or not a reaction will actually proceed is the oxidation of coal or diamond in air to form carbon dioxide. This reaction is very spontaneous (ΔG⦵ = − 395 kJ mol−1) but coal and diamond are stable in the atmosphere at normal temperatures for millions of years. To summarise: spontaneity is a thermodynamic property and gives information about the amount of energy that can potentially be produced to do work whereas whether or not a reaction actually occurs (i.e. the rate of the reaction) is a kinetic property and essentially depends upon the activation energy of the reaction.

 IB Docs (2) Team
IB Docs (2) Team