Determining Kc for an esterification reaction
 Background information
Background information
Equilibrium is an important topic in chemistry and one that needs to be understood by students. This particular practical covers equilibrium calculations covered under the AHL sub-topic 17.1 Equilibrium law but also reinforces chemistry that is covered in some of the core parts of the syllabus (e.g. 10.2 Alcohols and D.2 Aspirin & penicillin). It can be difficult in a school laboratory to measure the value of Kc as attempts to determine the concentrations of reactants and products can often disturb the position of equilibrium considerably. Probably for this reason there is no mandatory practical for Topics 7 & 17: Equilibrium. Even so, this makes a good practical as the rate at which ethanoic acid and ethanol react is quite slow (equilibrium at room temperature typically takes about one week to be reached). This means that titrating the mixtures for the amount of acid remaining (which effectively removes the ethanoic acid from the equilibrium mixture) has a negligible effect on the position of equilibrium. It can be useful to discuss other ways in which the concentration of reactants and products can be determined. One such way in which esterification can be followed is by gas liquid chromatography as the area under each of the peaks due to ethanol, ethanoic acid and ethyl ethanoate is directly proportional to their concentration in the mixture
This experiment has few hazards as long as students are careful when handling the pure ethanoic acid. It is called 'glacial' ethanoic acid because its melting point of 16.6 oC is only just below room temperature. If your laboratory is colder than this then the bottle will look as if it contains ice-crystals and may need warming before it can be used. Ethanol and ethyl ethanoate are also used in their pure form although 'pure' ethanol will probably be a 96% ethanol/4% water mixture. If you are unable to get hold of pure ethyl ethanoate then simply leave it out of the initial mixtures used as long as they still make a total volume of 10 cm3 The concentration of the hydrochloric acid is not crucial but should be about 3.0 mol dm-3. Because the calculations are quite involved and the literature value of Kc at 298 K can be obtained this is a good experiment to use as part of the 'scaffolding' to prepare students for their Individual Scientific Investigation as they can discuss uncertainties and experimental error. The questions and help on how to calculate the value of Kc will also help to reinforce the underlying theory. Versions of this esterification equilibrium practical have a long history of use for British A levels. For example, Salters have a pdf with a very full description including teacher's notes.
Introducing the topic prior to the experiment
Before starting this particular experiment it can be useful to demonstrate other equilibrium reactions (and therefore Le Chatelier’s Principle) without actually determining Kc. There are several tried and tested experiments that work for this. For example:
1. Placing 50 cm3 of nitrogen (IV) oxide in a sealed 100 cm3 gas syringe and altering the pressure.
2NO2(g) (dark brown) ⇌ N2O4(g) (colourless)
As the plunger is pushed in and the pressure increases the colour initially goes darker as the concentration of the NO2 increases. Then as the equilibrium re-establishes the colour lightens due to the formation of more dinitrogen tetroxide. Similarly when the plunger is pulled out and the pressure decreased it will initially go lighter but then darken as the mixture becomes richer in NO2.
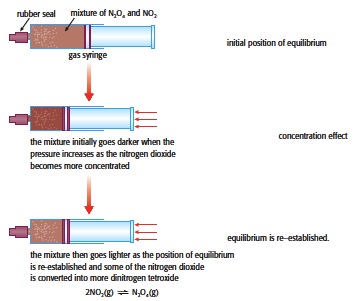
This is a nice experiment to explain to students the difference between the concentration effect and establishing equilibrium.
2. Although not strictly on the syllabus the colour changes for the equilibrium between chromate(VI) (yellow) and dichromate(VI) (orange) can also be demonstrated.
2CrO42-(aq) + 2H+(aq) ⇌ Cr2O72-(aq) + H2O(l)
Details for this can be found on the The Nuffield Foundation and Royal Society of Chemistry Practical Chemistry website but be careful to ascertain whether chromate(VI) can be legally used in your country as in some countries its use is banned in schools. If this is the case you can show the following video.
Chromate-dichromate equilibrium ![]()
3. An alternative equilibrium demonstration using transition metal ions is to change the ligands in copper(II) sulfate solution.
[CuCl4]2-(aq) (green) ⇌ [Cu(H2O)6]2+(aq) (pale blue) ⇌ [Cu(NH3)4(H2O)2]2+(aq) (intense blue)
If you still have access to an old OHP projector place a beaker containing a dilute solution of copper(II) sulfate on the projector and project the image onto a white screen. Add some concentrated ammonia solution dropwise. Initially a precipitate of copper(II) hydroxide will be seen but after a few more drops an intense blue solution of [Cu(NH3)4]2+ ions will be formed. If you then carefully add concentrated hydrochloric acid the reaction will be reversed and then proceed to give a green solution which mainly consists of [CuCl4]2- ions. Obviously be careful while you are doing this but you should get applause as white fumes of hydrogen chloride are evolved during the process. If you then add water the solution will revert back to the original blue colour of the hexahydrated copper(II) ions, [Cu(H2O)6]2+.
4. Another good example is to use a dilute solution of yellow iron(III) ions with thiocyanate ions to give the blood-red iron(III) thiocyanate complex ion.
[Fe(H2O)6]3+ (yellow) + SCN- ⇌ [Fe(SCN)(H2O)5]2+ (blood-red)
You might also wish to get students to use simulations to illustrate changes to systems in equilibrium.
 Student worksheet
Student worksheet
DETERMINING THE EQUILIBRIUM CONSTANT FOR AN ESTERIFICATION REACTION
The equilibrium constant for the acid-catalysed esterification of ethanoic acid with ethanol will be determined. This particular reaction is quite slow so it is possible to measure the concentration of a reactant by titration without significantly disturbing the equilibrium in the short space of time it takes to carry out the titration.
ENVIRONMENTAL CARE:
None of the reactants or products are damaging to the environment in the quantities used.
SAFETY:
Take care when handling the pure ('glacial') ethanoic acid.
PROCEDURE:
1. Make up four of the following mixtures using the pure liquids (water, ethyl ethanoate, ethanol and ethanoic acid) and the solution of hydrochloric acid provided in the burettes. Run the liquids into the reagent bottles to give a total volume of 10 cm3 then stopper immediately to prevent evaporation. Shake well then allow the bottle to stand at room temperature for one week to allow the mixture to reach equilibrium. Weigh separately 5 cm3 of each of the liquids used to make up the mixtures (including the HCl) and record the mass.
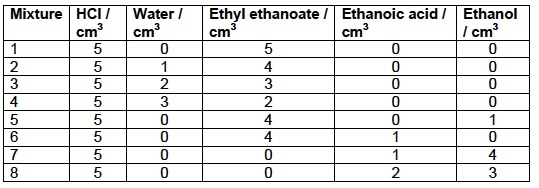
2. After one week titrate the whole of the mixture in each bottle with 1.00 mol dm-3 sodium hydroxide using phenolphthalein as the indicator. In order to find the exact concentration of the HCl catalyst, also titrate the approximately 3 mol dm-3 HCl(aq) with the 1.0 mol dm-3 sodium hydroxide solution. Record the results from another pair of students for the four mixtures you did not do.
CALCULATION
Give the balanced equation for the reaction between ethanoic acid and ethanol.
For each mixture
1. Calculate the amount (in mol) of ethyl ethanoate, ethanoic acid, ethanol and water present in the original mixture (i.e. before any reaction had taken place). When calculating this for the water remember to take into account the water present in the dilute HCl.
2. Calculate the amount (in mol) of ethanoic acid present in the equilibrium mixture and hence the amount (in mol) of ethanol, ethyl ethanoate and water in the equilibrium mixture.
3. Calculate the equilibrium concentrations of the water, ethyl ethanoate, ethanoic acid and ethanol in the mixture and hence the equilibrium constant.QUESTIONS
1. Comment on the values obtained for the eight different mixtures.
2. The accepted value for Kc at 298 K is 4.0. Calculate the percentage error and evaluate your experiment.
3. ΔH for this reaction is + 17.5 kJ mol-1. Would you expect the value of Kc to be greater or smaller at 60 oC than at room temperature?
4. The yield of ester formed in the reaction increases considerably if concentrated sulfuric acid is added to the reactants. Suggest a reason for this.
5. What other methods, apart from titration, could be used to determine equilibrium constants?
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team