Understanding the activity series
The problem - why is Li above K in the activity series?
There can be considerable confusion for standard level students over the position of certain metals in the activity series given in Section 25 the IB data booklet. One of the understandings in the IB Chemistry guide (Topic 9.1) states “The activity series ranks metals according to the ease with which they undergo oxidation.” Many searches online state that the activity series is the same as the reactivity series. Some (re)activity series place potassium at the top as the most reactive common metal whereas others put lithium at the top. So which is correct?
The reaction of alkali metals with water
.png) By looking at the reactions of alkali metals with water it seems obvious that potassium is actually more reactive than lithium. When potassium comes into contact with water it reacts quickly and exothermically to produce hydrogen and lilac sparks can be seen as it catches fire. Lithium reacts much more slowly and although hydrogen is steadily produced there are no sparks or flames to be seen. Many sources online state (wrongly!) that the reason why potassium is more reactive than lithium is because the outer electron that is being removed to form the ion is further from the nucleus in potassium than it is in lithium (i.e. in a higher energy level) so less energy is required to remove it. They go on to provide evidence for this by comparing the lower ionization energy of potassium (419 kJ mol-1) with that of lithium (520 kJ mol-1). So why is lithium placed above potassium in the IB’s activity series?
By looking at the reactions of alkali metals with water it seems obvious that potassium is actually more reactive than lithium. When potassium comes into contact with water it reacts quickly and exothermically to produce hydrogen and lilac sparks can be seen as it catches fire. Lithium reacts much more slowly and although hydrogen is steadily produced there are no sparks or flames to be seen. Many sources online state (wrongly!) that the reason why potassium is more reactive than lithium is because the outer electron that is being removed to form the ion is further from the nucleus in potassium than it is in lithium (i.e. in a higher energy level) so less energy is required to remove it. They go on to provide evidence for this by comparing the lower ionization energy of potassium (419 kJ mol-1) with that of lithium (520 kJ mol-1). So why is lithium placed above potassium in the IB’s activity series?
To answer this question we need to look more carefully at the reaction that actually takes place when the solid metal is added to water.
2M(s) + 2H2O(l) → 2M+(aq) + H2(g) + 2OH−(aq)
To use the value for the ionization energy correctly the solid metal must first be turned into the gaseous state (atomization energy). Once the gaseous ions are formed from the gaseous atoms (ionization energy) they then becomes hydrated to form the aqueous metal ions (hydration energy). From an energy cycle the overall enthalpy change for the reaction can be calculated. The following cycle gives the values for both lithium and potassium.

It can be seen that the atomization energy and the ionization energy are both more endothermic for lithium but the hydration energy is considerably more exothermic. It is this relatively high hydration energy that makes the overall enthalpy change less endothermic for lithium than for potassium. The higher hydration energy is due to the smaller size of the lithium ion compared to the potassium ion which increases its charge density.
(Although understanding and using the activity series is on the core part of the syllabus the next part of the explanation uses concepts from the Additional Higher Level part of the course.)
To obtain the value for the standard free energy change for the reaction, ΔG⦵, we would need to factor in the entropy change and use the expression ΔG⦵ = ΔH⦵ – TΔS⦵. When the reaction occurs in water we would also need to factor in the free energy change for the reaction of water to hydrogen and hydroxide ions. However ΔG⦵ is related to the standard electrode potential by the expression ΔG⦵ = − nFE⦵. Since the values for n and F, and the energy change for the formation of hydrogen and hydroxide ions are the same for both systems, the total EMF produced when the metals are added to water is directly proportional to the free energy.

Li(s)/Li+(aq) is higher in the electrochemical series than K(s)/K+(aq) so the reaction of lithium with water is more spontaneous and produces more free energy than the reaction of potassium with water.
E⦵(total) for Li(s) + H2O(l) → Li+(aq) + ½ H2(g) + OH–(aq)
= − 0.83 – (− 3.04) = + 2.21 V
E⦵(total) for K(s) + H2O(l) → K+(aq) + ½ H2(g) + OH–(aq)
= − 0.83 – (− 2.93) = + 2.10 V
This is why lithium is above potassium in the IB’s activity series as it follows the order of the metals in the electrochemical series (Section 24 in the data booklet). However it still does not explain why potassium reacts so much more vigorously than lithium when it is placed in water.
If we look at the values for the enthalpy change occurring with all the alkali metals when they react with water it is striking how very similar the values all are.

This illustrates the point that whether or not a reaction is spontaneous (i.e. ΔG⦵ has a negative value) it does not tell us anything about the rate of the reaction as that depends upon the activation energy. For example, the combustion of carbon in air is highly spontaneous and yet coal and diamond are very unreactive in air under standard conditions. From observation the rate of the reaction with water is very much faster for potassium than lithium. Consider the meting points of the alkali metals.
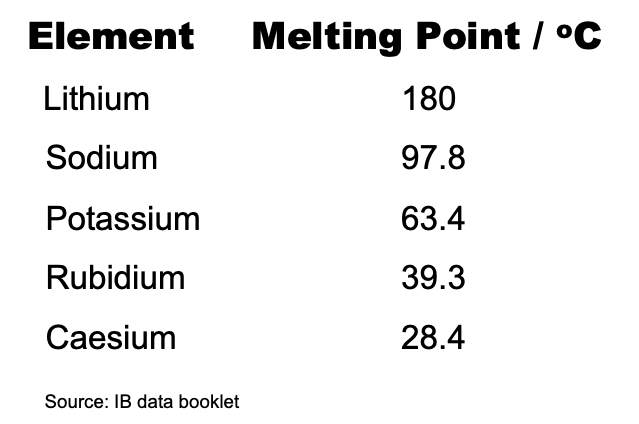
The melting points decrease down the group so the lower the melting point the more readily the heat produced turns the solid into molten metal and the surface area will rapidly increase. It may be for this kinetic reason that the rate of the reaction increases from lithium to caesium so approximately the same amount of energy is given out but in less time. The Gold Book which is one of the most authoritative glossaries of chemistry does not list 'activity series' or 'reactivity series' but under 'activity' it states “As applied to a chemical species, the term reactivity expresses a kinetic property.” I would suggest that it is correct to place lithium at the top of the activity series for metals, as the series is based on thermochemical properties. However, as reactivity series are based on kinetic properties, potassium should be placed at the top of the reactivity series for metals.
Just to finish this off you might like to try to answer the following question.
Why is the reaction of alkali metals with water so exothermic when the process of M(s) → M+(aq) + e− is endothermic and breaking the O−H bond (463 kJ mol-1) requires more energy than is given out when the H−H bond (436 kJ mol-1) is formed?The main factor is the hydration energy given out when the hydroxide ion is formed from the water. The IB data book gives a value of − 519 kJ mol-1 for the hydration energy of the hydroxide ion.

 IB Docs (2) Team
IB Docs (2) Team