Opiates

 D.3 Opiates (2 hours)
D.3 Opiates (2 hours)
Pause for thought
Some care needs to be taken with some of the statements made in the subject guide about morphine and its derivatives.
In the Nature of Science section for D.3 it states
“Data and its subsequent relationships—opium and its many derivatives have been used as a painkiller in a variety of forms for thousands of years. One of these derivatives is diamorphine“
This implies to me that diamorphine (heroin) has been known and used for ‘thousands of years’. The opium poppy (Papaver somniferum) does naturally contain over 40 different alkaloids. Most prominent amongst these is morphine (about 12 - 21%) with the second most prominent being codeine (0.5 - 2.5%). Morphine was first identified chemically in 1805 by Friedrich Sertürner (1783-1841) and it was used as a pain-killer in the American War of Independence. Codeine was isolated from opium three decades later in 1832 by Pierre Robiquet (1780 – 1840), a French chemist. However diamorphine (heroin) is semi-synthetic and is not present naturally in the opium poppy. It was first synthesised from morphine in the same decade (the 1890s) and by the same company that first synthesised aspirin – the Bayer Company in Germany. It certainly has not been known for ‘thousands of years’.
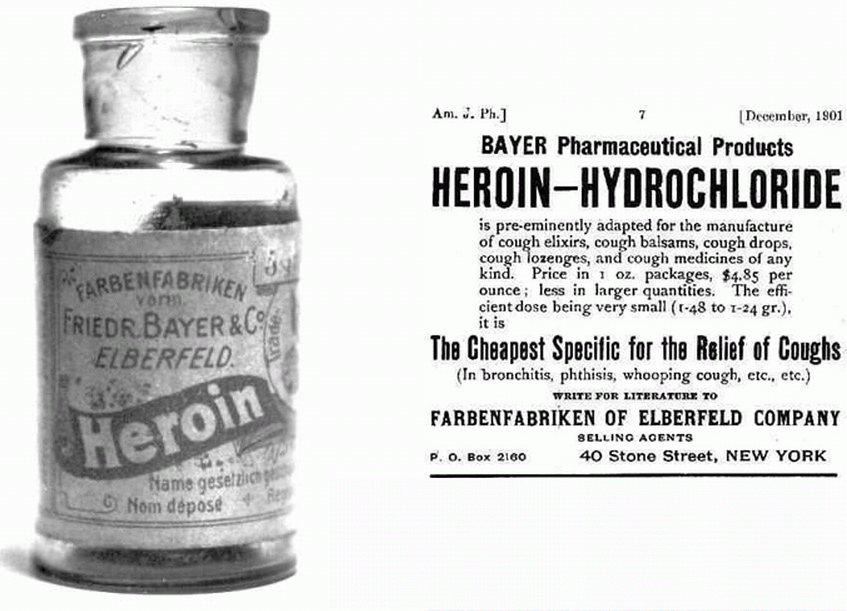
The first statement in the ‘Application and skills’ section is “Explanation of the synthesis of codeine and diamorphine from morphine. Codeine of course does not have to be synthesised from morphine as it does occur naturally however because it only occurs naturally in relatively small mounts much that is produced industrially is synthesised from morphine. I have included a good video from the Royal Society of Chemistry in the ‘other resources’ section below that gives a very thorough explanation as to how the phenol group is substituted for an ether group. Essentially it involves using iodomethane as the reagent in a nucleophilic substitution reaction. The synthesis of diamorphine from heroin is very similar to the synthesis of aspirin from salicylic acid as ethanoic anhydride is used in both cases in preference to just ethanoic acid, as it is a better nucleophile. The only major difference is that the synthesis of diamorphine is an example of diesterification rather than monoesterification.
Nature of Science
Opium has been used as a painkiller for thousands of years. Chemists have adapted this natural substance to produce semi-synthetic derivatives (e.g. diamorphine) with similar properties.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe structures of morphine, codeine and diamorphine (heroin) can be found in Section 37 of the data booklet. International-mindednessMany illegal drugs, including opiates, are grown and/or produced in a relatively small number of countries and then trafficked and sold worldwide. |
Teaching tipsShow students the structures of morphine, codeine and heroin and get them to realise that the basic structure is identical with the only difference being due to one or two of the functional groups. I like to then ask students how they would change morphine into heroin (if they lived in Afghanistan!). The answer they should give is simply warm with ethanoic acid as it is just a straightforward diesterification reaction (in reality you would actually use ethanoic anhydride as it is a better nucleophile). You can also ask them to predict which out of morphine or heroin will be more polar and then use their answer to explain that heroin can cross the lipid-based blood/brain barrier to reach the brain quicker and in higher concentrations than morphine. They should also be able to see that the structures contain a tertiary amine group. This can readily be converted into a salt by reaction with an acid and they should realise that morphine and heroin are usually administered as a salt to aid bioavailability. It might be worth asking a drug counsellor or other health professional to visit the school to talk about the social and economic problems associated with heroin addiction. | Study guide
Page 157 QuestionsFor ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Opiates. For short-answer questions see Opiates questions together with the worked answers on a separate page Opiates answers. Vocabulary listmorphine |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. An excellent video from the Royal Society of Chemistry explaining how codeine can be semi-synthesised from morphine by nucleophilic substitution.
![]() Synthesis of codeine from morphine
Synthesis of codeine from morphine
2. Another good video on morphine and heroin from Nottingham University which also explains its chemistry.

 IB Docs (2) Team
IB Docs (2) Team 















