Preparation of 1,3-dinitrobenzene
 Introduction
Introduction
This practical is really only for Higher Level students as it is an example of an electrophilic substitution reaction and covers the syllabus content for electrophilic substitution listed in 20.1 Electrophilic substitution.
The syllabus actually requires the nitration of benzene to give nitrobenzene as the example of electrophilic substitution. This cannot be done practically in the laboratory as the use of benzene is banned in schools due to its potential carcinogenic nature.
When benzene is nitrated a mixture of concentrated nitric acid and sulfuric acid is required but the reaction goes quite readily. The temperature should be kept below 50 oC in order to prevent further nitration occurring. The electrophile is the nitronium ion, NO2+ formed when the sulphuric acid protonates the nitric acid:
HNO3 + H2SO4 → H2NO3+ + HSO4−
H2NO3+ → NO2+ + H2O
The product nitrobenzene, C6H5NO2, is less reactive towards further nitration as the nitro- group is electron withdrawing making the benzene ring less susceptible to attack by electrophiles. This practical starts with pure nitrobenzene and in order for the reaction to occur it needs to be heated under reflux for at least twenty minutes.
Teacher's notes
This experiment gives students a good experience of organic preparative chemistry as once the reflux is complete the students need to precipitate the product by changing this polarity of the solution. This is done by adding water. They also get experience of recrystallisation as the solid 1,3-dinitrobenzene is then recrystallised from ethanol. After drying and weighing they can test the purity of their product by comparing the melting point with the literature value of 88-90 oC and also perform thin layer chromatography. You should be aware that sometimes students can end up with an oily mixture that cannot be satisfactorily recrystallised. Several years ago a student of mine did their extended essay on this and discovered that for the reaction to work with certainty the concentrated nitric acid used should preferably be freshly prepared or at least taken from a new bottle. Care, of course, should be taken whenever concentrated sulfuric and nitric acids are used and safety glasses must be worn at all times.
In the current programme there is no need to explain why nitrobenzene is less reactive towards electrophiles than benzene or why it is 3- directing. However good students can easily read about this for themselves as it is good chemistry and not difficult to understand. Similarly the use of chromatography is not on the core or AHL part of the programme but it is a good technique and students should at least have some working knowledge of it even if they are not going to come across it in the particular option they choose.
 Student worksheet
Student worksheet
PREPARATION OF 1,3-DINITROBENZENE
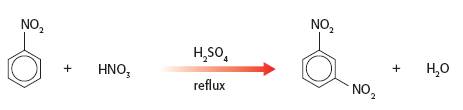
Nitrobenzene can be nitrated to 1,3-dinitrobenzene by heating with nitric acid in the presence of sulfuric acid. The product is obtained as fine pale yellow crystals. This experiment exemplifies the technique of purification by recrystallisation and illustrates the use of melting points and chromatography to determine the purity of the product.
ENVIRONMENTAL CARE:
To minimise cost and the effect on the environment the procedure given below is for the small-scale preparation of 1,3-dinitrobenzene. It involves considerably less chemicals than the traditional practical but does mean that care is required at all stages of the preparation to obtain a good yield. All organic waste should be disposed of in the appropriate container in the fume cupboard.
SAFETY:
Safety glasses must be worn when using concentrated acids. As much as possible avoid breathing in the fumes of nitrobenzene.
PROCEDURE:
Using a small measuring cylinder and a teat pipette carefully transfer 1.5 cm3 of fresh concentrated nitric acid and then 2 cm3 of concentrated sulfuric acid to a 10 cm3 round-bottom 'Quickfit' flask. Place the flask on a top pan balance and using a new dropping pipette carefully add about 1.5 g of nitrobenzene and record the exact weight.
Fix an air condenser to the flask, swirl to ensure mixing of the reagents, and place in a beaker of boiling water. Reflux the mixture for 20 minutes swirling frequently. After twenty minutes, remove the water bath and allow to cool for five minutes.
Pour the contents of the flask into a beaker containing 25 cm3 of tap water. Rinse out the residue of the flask with a little water if necessary. Allow the mixture to stand for five minutes and stir occasionally. Filter the contents through a sintered glass crucible attached to a Buchner flask and water pump. Wash the solid product with a little water to remove the last traces of acid. Suck dry, and transfer the solid to a weighed watch glass. Dry the product overnight and reweigh to determine the yield of crude 1,3- dinitrobenzene.
Meanwhile take a small quantity of the crude product and place in a 10 cm3 test-tube. Add no more than 1 cm3 of ethanol and then warm the mixture in a hot water bath until you obtain a clear solution. Allow to cool. Filter the crystals obtained through the sintered glass crucible under reduced pressure. Wash with a little freezer-cooled ethanol and dry the crystals. Measure the melting point of the crystalline 1,3-dinitrobenzene and compare your value with the value given in the data book. To test the purity of your product dissolve a few crystals in a few drops of trichloromethane. Place a spot of this solution 1 cm from the base on a microscope slide coated in silica. Place the slide in a jar containing a 50:50 mixture of trichloromethane and propanone as the eluent. Replace the lid on the jar and allow the eluent to rise up the slide. Mark the level reached by the eluent and then let the slide dry. Develop the chromatogram by placing the slide in another jar containing a few crystals of iodine.
QUESTIONS
1. Give the equation for the preparation of 1,3-dinitrobenzene and discuss the mechanism for the reaction.
2. State two ways in which the melting point of an impure substance may vary from that of the pure substance. What is meant by a 'mixed melting point determination'?
3. In the final part of the practical you have used tlc (thin layer chromatography). Write brief notes on other types of chromatography in particular column chromatography, paper chromatography and glc (gas-liquid chromatography). What is meant by the Rf value?
This worksheet can also be downloaded from:

 IB Docs (2) Team
IB Docs (2) Team