Example of connections
Chlorination of methane
This example looks at how the reaction of methane with chlorine can be used to illustrate several different topics. A gallery of slides covering the concepts together with equations and relevant data values for use with your students is provided at the foot of the page.
Organic context
One reaction that we all cover thoroughly is the chlorination of methane which is listed in sub-topic 10.2 Functional group chemistry for organic chemistry.
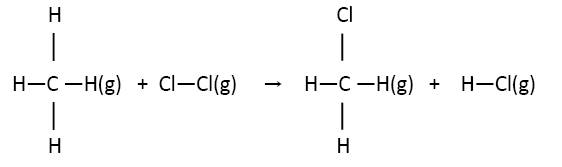
We then go on to teach the free radical substitution mechanism breaking the reaction down into the three phases of initiation, propagation and termination.
Step 1. Homolytic fission of Cl–Cl bond by ultraviolet light
Cl2 → 2Cl.
Step 2. Propagation of radicals
CH4 + Cl. → H–Cl + CH3.
CH3. + Cl2 → CH3 Cl + Cl.
Step 3. Termination
e.g. CH3. + Cl. → CH3 Cl
Finally we may talk about the fact that the substitution can go all the way to form tetrachloromethane.
Fine. But why only use this example when covering topic 10. What about relating it to some of the other topics?
For example, we could look at the energetic of the reaction.
Energetics context
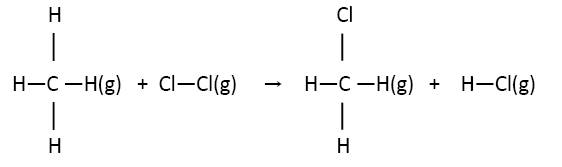
Using average bond enthalpy values from Section 11 of the IB Chemistry Data Booklet we can work out the enthalpy change for the reaction:
Energy in: C–H + Cl–Cl = 414 + 242 = 656 kJ
Energy out: C–Cl + H–Cl = 324 + 431 = 755 kJ
∆H![]() = – 99 kJ mol-1
= – 99 kJ mol-1
Clearly the reaction is exothermic. We also know it is fast (as it is a free-radical reaction) and the reactants and products are gases at room temperature and not too heavy. How does this compare with the combustion of hydrogen?
O=O(g) + 2H–H(g) → 2H2O(g)
Energy in: O=O + 2H–H = 498 + (2 x 436) = 1370 kJ
Energy out: 4O–H = (4 x 463) = 1852 kJ
∆H![]() = – 482 kJ (or -241 kJ mol-1 for hydrogen)
= – 482 kJ (or -241 kJ mol-1 for hydrogen)
This shows that the combustion of hydrogen is more exothermic. Hydrogen and oxygen have been used as rocket fuels as the reaction is so fast and exothermic. How could we make the halogenations of methane more exothermic? We need a reactant with weak bonds and a product with strong bonds. What would happen if we replace chlorine with fluorine? The F–F bond is weaker than the Cl–Cl bond and the C–F bond is stronger than the C–Cl bond.
CH4(g) + F2(g) → CH3F(g) + HF(g)
Energy in: C–H + F–F = 414 + 159 = 573 kJ
Energy out: C–F + H–F = 492 + 567 = 1059 kJ
∆H![]() = – 486 kJ mol-1
= – 486 kJ mol-1
Wow! That is considerably more exothermic. Perhaps rocket scientists should consider methane and fluorine as a rocket fuel!
How about also relating the chlorination of methane to sub-topic 9.1 Oxidation & reduction?
Oxidation and reduction context
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
Oxidation states: C in CH4 −4, Cl in Cl2 0, C in CH3Cl −2, and Cl in CH3Cl and in HCl −1
Carbon has been oxidized (from −4 to −2) and chlorine has been reduced (from 0 to −1).
This means according to the first statement listed under 'Understandings' in sub-topic 9.1 Oxidation & reduction it is a redox reaction
"Oxidation and reduction can be considered in terms of oxygen gain/hydrogen loss, electron transfer or change in oxidation number".
So we should be able to write the half-equations for this reaction.
Half-equations?
Cl2 + 2e− → 2Cl−
CH4 → CH3+ + H+ + 2e−
Overall: CH4 + Cl2 → CH3Cl + HCl
But this introduces a problem. How does this ‘ionic interpretation’ square with the free radical mechanism? It is a good example of TOK. The mechanism is not ionic and although the carbon has been oxidised it has not lost any electrons so writing half-equations is meaningless. You can read more about this problem of the definition of oxidation in the TOK section on How useful are oxidation states?
Hopefully you can see from this example that by taking one reaction which we normally ‘pigeon hole’ within only one of the eleven IB topics and looking at it in a different context in some of the other ten topics can lead to some interesting chemistry. Moreover it increases the understanding of students and their ability to think critically and make less obvious links.
I've added the powerpoint below which takes you through this stepwise which you could use in your teaching.

 IB Docs (2) Team
IB Docs (2) Team 














































