Novel uses for the IB data booklet

As well as providing essential data, the IB data booklet can be used as a great teaching aid to introduce and reinforce some of the fundamental concepts covered in the course.
Using the IB data booklet as a teaching resource
The IB chemistry data booklet is used when the students take the Paper 2 and Paper 3 examinations at the end of the course. Clearly students need to be completely familiar with its contents well before this so it is good practice to give the students their own copy right at the beginning of their two year course. As well as being a source of data it can be a very useful teaching aid in its own right.
Many teachers and students use it only as it was originally intended. For example, Section 6 is the standard Periodic Table for use throughout and relevant sections are used when covering individual topics. Thus Section 21 – Strengths of organic acids and bases and Section 22 – Acid-base indicators are referred to when covering Topics 8 and 18 etc. However it can be of much more use than this.
Since students have their own copy and should get into the habit of bringing it to class it can be an extremely useful resource which is immediately available for the teacher to illustrate many different concepts. It can add considerable relevance, interest and context to otherwise relatively dry theoretical subject content. Even if they are not studying options such as Biochemistry or Medicinal chemistry students are interested in the substances contained in these options and can relate directly to them. Through these ideas students can get a much broader perspective of chemistry and increase their general understanding.
It is also worth noting that questions on Paper 1, Paper 2 and Paper 3 may use information from the IB data booklet to illustrate certain concepts. For example:
An example of the use of a structure from an option being used in a multiple choice question on the core.
Which reactions give a negative ΔH value?
I. 2H2(g) + O2(g) → 2H2O(l)
II. C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
III. The reaction between sodium hydroxide and aspirin.
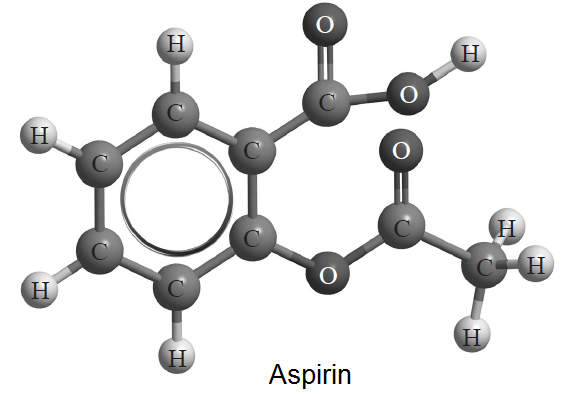
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
The concepts tested in Papers 1 and 2 will of course be on the core or AHL but the examples used may be taken from the sections covering the options. In addition to the example above on energetics/thermochemistry, students might be asked to identify functional groups in molecules whose structures are given in Sections 34, 35, or 37 of the data booklet or deduce whether a given molecule listed in one of these tables is polar or chiral. Similarly there could be a question on Paper 1 or 2, for example, which tests knowledge of infrared, or 1H NMR spectroscopy using a molecule from the Biochemistry or Medicinal chemistry options.
I have provided details of some different innovative ways in which the data booklet can be used successfully to illustrate and support the teaching of particular concepts.
1. ![]()
![]() Shapes of molecules and hybridization
Shapes of molecules and hybridization
2. ![]() Enantiomers
Enantiomers
4. ![]()
![]() Classes of compounds & functional groups
Classes of compounds & functional groups
5. ![]()
![]() Solubility
Solubility

 IB Docs (2) Team
IB Docs (2) Team