D.4 pH regulation of the stomach
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Option D - sub topic D.4. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.


 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand
Understand
- Antacids reduce excess stomach acid.
- The active form(s) of a drug after it has been processed by the body are called active metabolites.
Apply your knowledge to:
- Explain how excess acidity in the stomach can be reduced by the use of different bases.
- Construct and balance equations for neutralization reactions and apply theses equations using their stoichiometry.
- Solve buffer problems using the Henderson–Hasselbalch equation.
- Explain how compounds, such as ranitidine (Zantac), can be used to inhibit stomach acid production.
- Explain how compounds, such as omeprazole (Prilosec) and esomeprazole (Nexium), are used to suppress acid secretion in the stomach.
Relationships & vocabulary
Nature of science
The symptoms of dyspepsia include the overproduction of stomach acid. There are several different ways in which this can be treated. These includes the prescription of antacids to neutralize the acid, the use of H2-receptor antagonists and the use of proton pump inhibitors which work by preventing the production of stomach acid. The effectiveness of these different treatments can be assessed through collecting data by sampling and trialling.International-mindedness
The need for pH regulation of the stomach can be affected by the diet, lifestyle and genetics etc. of different cultures.Vocabulary
| dyspepsia | antacid | metabolite | Henderson-Hasselbalch equation |
| ranitidine | omeprazole | esomeprazole | H2 receptor antagonist |
| proton pump inhibitor |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
Parts of this sub-topic could be a real problem if you are a Standard Level student - somehow there seems to be a mismatch between the content in the core topics and the content of the core part of the option. In Topic 8: Acids and bases, which forms part of the main core, you do cover the definition of pH, but you do not cover acid or base ionization constants, Ka or Kb, or pKa or pKb, or the relationship between pKa and pKb. Neither do you learn anything about buffer solutions, nor pH curves so you will are not familiar with the half-equivalence point. In sub-topic 18.3, even Higher Level students are not required to solve buffer calculations and yet in this core sub-topic, Standard Level students as well as Higher Level students are expected to solve problems involving buffer solutions by using the Henderson-Hasselbalch equation.
Because you will always have access to the data booklet in the examination for this option you do not need to remember the Henderson-Hasselbalch equation. However to expect you to apply this equation without any understanding seems contrary to the good teaching the IB expects. In fact, in the past I have never used the equation as such as I prefer students to always work from first principles rather than learn or use an unnecessary equation. All you need to know to solve buffer calculations is that pH = −log10 [H+(aq)] (Topic 8) and from this you can deduce that pKa = −log10 Ka. You also need to know that for a weak acid in water HA(aq), the acid dissociation constant, Ka can be expressed just like any other equilibrium constant (Topic 6), i.e.
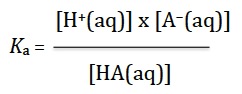
Although not necessary to solve problems, the Henderson-Hasselbalch equation can easily be derived from this.
If logarithms to the base ten are then taken the equation becomes
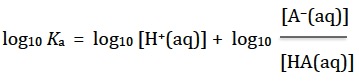
Subtract log10 Ka and log10 [H+(aq)] from both sides so it becomes
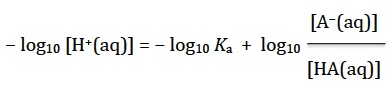
Substituting the definitions for pH and pKa gives the normal expression for the Henderson-Hasselbalch equation
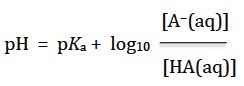
I suspect (hope?) that for Standard Level students the calculations will mainly focus on the special case when the concentration of the salt is equal to the concentration of the acid, i.e. at the half-equivalence point when a weak acid is titrated with a strong base. At this point [A−(aq)]/[HA(aq)] = 1, so log10 ([A−(aq)]/[HA(aq)]) = zero and the pH = pKa.
In fact you should realise that there is no need to use the Henderson-Hasselbalch equation at all to arrive at this conclusion as it comes directly from the equilibrium expression for the acid dissociation. When [A−(aq)] = [HA(aq)] then Ka is simply equal to [H+(aq)] and hence pH = pKa.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: pH regulation of the stomach.
For short-answer questions see pH regulation of the stomach questions together with the worked answers on a separate page pH regulation of the stomach answers.
More resources
1. A helpful article from Patient UK which explains what antacids are and how they work.
2. A rather nice explanation, including an animation, of how ranitidine works by binding to the H2-histamine receptor.
3. Another animation to show how proton pump inhibitors work.
![]() Action of proton pump inhibitors
Action of proton pump inhibitors

 IB Docs (2) Team
IB Docs (2) Team 






















