4.4 Intermolecular forces
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Topic 4.4 Intermolecular forces. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)

 Learning outcomes
Learning outcomes
.png) After studying this topic you should be able to:
After studying this topic you should be able to:
Understand:
- London (dispersion) forces, dipole-dipole forces and hydrogen bonding are all included in intermolecular forces.
- In terms of their relative strengths the order of these interactive forces are London (dispersion) forces < dipole-dipole forces < hydrogen bonds.
Apply your knowledge to:
- Deduce the types of intermolecular force present in substances, based on
the structure and chemical formula of the substance. - Explain the physical properties of covalent compounds (to include volatility, electrical conductivity and solubility) in terms of their structure and intermolecular forces.
Relationships & vocabulary
Nature of Science
Evidence for scientific theories can be obtained by making and testing predictions based on them. For example, London (dispersion) forces and hydrogen bonding can be used to explain specific interactions. Since molecular covalent compounds can exist in the liquid and solid states there must be attractive forces between their particles which are significantly greater than those due to gravity alone.
International-mindedness
For examples and links to International mindedness, Theory of knowledge, utilization etc. see separate page which covers all of Topics 4 & 14: Chemical bonding & structure.
Vocabulary
| induced dipole | London (dispersion) forces | van der Waals' forces | dipole-dipole interactions |
| hydrogen bonding | intramolecular | intermolecular |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
1. What’s in a name?
Clearly there are attractive forces between all molecules and even between individual noble gas atoms. Helium has an extremely low melting point (-272 oC) but nevertheless it does freeze so some force must be holding helium atoms together below this temperature. Most books explain this by referring to the formation of ‘temporary dipoles’. Many give quite elaborate diagrams to show how at any one instant the electrons in one molecule are not evenly spaced so produce a very short-lasting dipole which causes it to be attracted to another molecule with a similar temporarily induced dipole. It is an appealing explanation but as far as I am aware no-one has ever actually proved it to be true. They have given this force a name though – the problem is that not everyone agrees on which name to give it. In the past the IB programme called it van der Waals' forces whereas on this new programme they call it 'London (dispersion) forces'. In fact both are correct.
 ‘van der Waals forces’ is a generic term. Technically it refers to any force present between molecules other than those due to much stronger covalent bonds or electrostatic interactions between ions (ionic bonds). London dispersion forces (which are very weak) are more specific and refer only to the forces of attraction between two non-polar molecules due (presumably) to temporary induced dipoles. They are named after Fritz Wolfgang London (1900 –1954) an American physicist. A specific name is also given to the slightly stronger van der Waals' attractive forces between a polar molecule and a non-polar molecule. This is known as a Debye force and is named after a Dutch physicist, Petrus Josephus Wilhelmus Debye (1884-1966). Stronger still van der Waals forces are present between molecules (such as propanone molecules) which possess a permanent dipole. The specific name for these is a Keesom force. This is named after Willem Hendrik Keesom (1876 –1956) who like Debye and Johannes Diderik van der Waals (1837– 1923) was also a Dutch physicist. Technically there is yet another even stronger van der Waals force of attraction known as hydrogen bonding but this is almost always referred to by its own name. Recently IUPAC has changed the way in which a hydrogen bond is defined to include some weaker interactions not necessarily involving fluorine, nitrogen or oxygen so even this can be quite confusing (see my blog on Hydrogen bonding).
‘van der Waals forces’ is a generic term. Technically it refers to any force present between molecules other than those due to much stronger covalent bonds or electrostatic interactions between ions (ionic bonds). London dispersion forces (which are very weak) are more specific and refer only to the forces of attraction between two non-polar molecules due (presumably) to temporary induced dipoles. They are named after Fritz Wolfgang London (1900 –1954) an American physicist. A specific name is also given to the slightly stronger van der Waals' attractive forces between a polar molecule and a non-polar molecule. This is known as a Debye force and is named after a Dutch physicist, Petrus Josephus Wilhelmus Debye (1884-1966). Stronger still van der Waals forces are present between molecules (such as propanone molecules) which possess a permanent dipole. The specific name for these is a Keesom force. This is named after Willem Hendrik Keesom (1876 –1956) who like Debye and Johannes Diderik van der Waals (1837– 1923) was also a Dutch physicist. Technically there is yet another even stronger van der Waals force of attraction known as hydrogen bonding but this is almost always referred to by its own name. Recently IUPAC has changed the way in which a hydrogen bond is defined to include some weaker interactions not necessarily involving fluorine, nitrogen or oxygen so even this can be quite confusing (see my blog on Hydrogen bonding).
To try to bring some simplification the IB specifies which nomenclature should be used (an example of language in Chemistry). Debye and London forces are both referred to by the IB simply as 'London (dispersion) forces' of attraction and Keesom forces are known as dipole-dipole interactions. Hydrogen bonding is reserved for the specific stronger dipole-dipole interactions between hydrogen bonded directly to fluorine, oxygen or nitrogen atoms in one molecule and the fluorine, oxygen or nitrogen atoms bonded to hydrogen in another molecule. Sometimes this can occur within the same molecule, then it is known as intramolecular hydrogen bonding. For example, consider hydroquinone and catechol.

benzene-1,4-diol (hydroquinone) - boiling point 287 oC

benzene-1,2-diol (catechol) - boiling point 245 oC
Intramolecular hydrogen bonding is not possible in hydroquinone as the two -OH groups are too far apart to interact so there is stronger intermolecular hydrogen bonding between the molecules. In catechol there will be some intramolecular hydrogen bonding between the hydrogen atoms on one of the phenolic groups with the oxygen atoms on the other phenolic group. This means the intermolecular forces of attraction will be weaker between the molecules so the boiling point is lower.
2. Solubility
In sub-topic 4.4 you are asked to compare the properties of covalent substances resulting from different types of bonding. This includes solubility in polar and non-polar solvents. Students tend to be fond of the 'like dissolves like' concept. Thus alkanes, which are non-polar, are immiscible with water but dissolve in non-polar solvents as they can form London (dispersion) forces of attraction with the non-polar solvent molecules. Of course, they could also form London (dispersion) forces of attraction with water molecules but the polar water molecules prefer to attract each other through hydrogen bonding. Polar molecules such as ethanol and propanone are miscible with water because the hydrocarbon part of the molecule is small. As the length of the carbon chain increases in the homologous series of alcohols the solubility in water rapidly decreases so that butan-1-ol is only slightly soluble in water and pentan-1-ol is hardly soluble at all.
 | 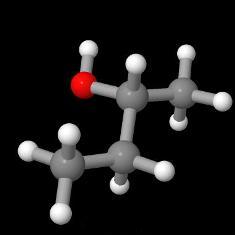 |  |  |
| butan-1-ol | butan-2-ol | pentan-1-ol | pentan-2-ol |
Note that as the molecule becomes more spherical the London (dispersion) forces of attraction between molecules becomes less, so butan-2-ol (290 g dm-3) is more soluble in water than butan-1-ol (63.2 g dm-3) and pentan-2-ol (45 g dm-3) is more soluble in water than pentan-1-ol (22 g dm-3).
All this is fine, but what about ionic compounds?
Since ions are obviously charged it would be reasonable to assume they will dissolve in a polar solvent such as water and of course virtually all the salts of the alkali metals, such as sodium chloride, are very soluble in water. I like to ask my students to explain why sodium chloride has a high melting point (801 oC) and they give the correct answer that considerable energy needs to be provided to overcome the lattice enthalpy. Fine - then I ask them why it dissolves easily in water, which also breaks the crystal structure and yet the temperature hardly changes (in fact it does drop very slightly). It makes them realise how strong the enthalpy of hydration is - for Higher Level students this is covered in 15.1 Energy cycles. The exothermic hydration enthalpy for sodium and chloride ions is only slightly less than the endothermic lattice enthalpy so by making up the difference by taking a small amount of heat from the water sodium chloride can dissolve. Once you move to group 2 salts though the lattice enthalpies get considerably larger. So do the hydration enthalpies as the smaller more highly charged cations attract water molecules more strongly. For some group 2 salts such as magnesium sulfate the difference is still small enough for the salt to be soluble but for others such as barium sulfate the difference is too much and the salt is essentially insoluble in water.

One final point worth stressing is that if there is no lattice enthalpy to overcome, the hydration enthalpy can be given off as a large amount of heat - for this reason you should never add water to concentrated sulfuric acid in order to dilute it. The image on the right shows the correct way to do it, although even then you should still take considerable care.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' multiple choice questions with the answers explained see MC test: Intermolecular forces.
For short-answer questions see Intermolecular forces questions.
More resources
1. Relating inter- and intra- molecular attractions to basketball. Two teachers, Jonathan Bergman and Aaron Sams talk about all the different types of intermolecular attractive forces.
2. A very short but simple video showing how hydrogen bonding arises in water and the properties that depend upon it.
3. A tutorial on intermolecular forces with some good questions from the University of Waterloo, Canada.

 IB Docs (2) Team
IB Docs (2) Team 
















