Topics 10 & 20
10 (
 11 h) & 20 (
11 h) & 20 ( 12 h) : Organic chemistry
12 h) : Organic chemistry
Possible ways of teaching organic chemistry
Organic chemistry is one of the bigger topics in terms of time and content. It takes up 11 of the 95 hours of Core time and 23 of the 155 hours allotted to the Core and AHL for Higher Level students. You will need to plan carefully how and when you intend to teach it. There are several different approaches that teachers have used successfully in the past. Some just plough through it as it is set out in the programme. There is much to be said for this in terms of simplicity but it does become somewhat of a memory exercise for your students as it can be considered as a long list of facts and reactions with not a great deal of underlying logic. In an attempt to introduce some logic to the content the core does contain one reaction mechanism - the free radical substitution of alkanes with halogens in ultra-violet light. Three more mechanisms are covered in the AHL . The nucleophilic substitution of halogenoalkanes (both SN1 and SN2), the electrophilic addition reactions of alkenes (including an explanation of Markovnikov's rule) and the electrophilic substitution reactions of arenes, although this is limited just to the nitration of benzene. Once the mechanism is understood then what was once a seemingly unrelated list of reactions such as the reactions of hydroxide ions, water, ammonia, amines and cyanide ions with halogenoalkanes becomes in fact just one reaction - the nucleophilic substitution of halogenoalkanes by nucleophiles. I personally favour this approach and rather regret the loss of the old option G on Further Organic Chemistry. Students in the past who covered this option were well prepared to study chemistry and medicine at university and this current 2014 programme actually contains considerably less organic chemistry content than previous programmes. What many teachers do is break up the teaching of core and AHL organic chemistry into two or more manageable portions and place a topic such as acids and bases in-between. I usually start teaching organic chemistry at the beginning of the second year. It is important to have covered the essentials of atomic theory and bonding and, for AHL, preferably some kinetics before starting the organic. Students will also benefit from having some knowledge of redox and acid base chemistry before learning about the chemistry of alcohols and carboxylic acids. I find it definitely helps to give students regular tests once you have completed the chemistry of certain functional groups otherwise they tend to get it all jumbled up if they just have one mega test at the end. Partly for this reason I have split sub-topic 10.2 : Functional group chemistry (which contains 6.5 hours of teaching) into two separate pages. The first part contains alkanes, alkenes and addition polymers and the second part contains alcohols, halogenoalkanes and benzene. I have also split the tests attached to these pages into separate functional groups so they can be given as and when the students have covered a particular functional group. There are good practicals to illustrate the underlying chemistry. However some of these are test-tube reactions or laboratory preparations. These involve useful chemistry techniques like reflux, recrystallisation, melting point determinations etc. but these are not Mandatory laboratory components for this topic. The only mandatory 'practical' is 3-D modelling of organic compounds. If each pair of students can have access to a good set of models (such as Molymod) throughout your teaching of the topic then this will considerably enhance their understanding. As you go through the topic, use the examples I have given from Novel uses for the IB data booklet . These provide interesting molecules that contain different functional groups to complement the usual examples of carbon compounds containing up to six carbons atoms. This will increase students' enjoyment as they will see the relevance of the topic and it will most probably help them when they come to study their chosen option. For example:
Is cholesterol a primary, secondary or tertiary alcohol?
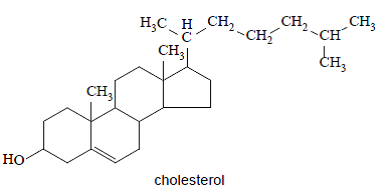
Secondary
Why organic chemistry is special
The first sub-topic has the heading "Fundamentals of organic chemistry" but in some ways it does not really cover why the chemistry of just one element, carbon, has a whole branch of chemistry all to itself. I think it is important that students not only know that there are more carbon compounds known than the total of all the compounds of all the other 100+ elements put together but why this is so. So my introductory lesson mentions the importance of Friedrich Wöhler and I stress the ability of carbon to form chains and rings and also the relative strength of the C-H bond compared to the Si-H, Ge-H and Sn-H bonds and the relative strengths of the carbon to carbon bonds (C-C, C=C and CΞC) compared to the bonds formed between the other Group 14 elements. I also like to explain to Higher Level students why tetrachloromethane just forms two immiscible layers when mixed with water whereas tetrachlorosilane (silicon tetrachloride) react vigorously with water. In fact organic chemistry provides a useful tool to reinforce many of the concepts that students have already met. How many teachers stress that many organic reactions are in fact redox reactions as there is a change in the oxidation state of carbon during the course of the reaction? Stress also, to Higher Level students, that organic reaction mechanisms involving the use of curly arrows (which represent the movement of pairs of electrons ) are examples of Lewis acid & base reactions.
The links on the left give you teaching tips etc. for each of the sub-topics together with questions and answers for each sub-topic.
Once you have finished teaching the whole topic you can give the multiple choice tests on Organic chemistry (together with the answers):
![]()
![]() Topic 10. Organic chemistry (1) and/or
Topic 10. Organic chemistry (1) and/or ![]()
![]() Topic 10. Organic chemistry (2)
Topic 10. Organic chemistry (2) ![]()
![]() Topics 10 & 20. Organic chemistry (1) and/or
Topics 10 & 20. Organic chemistry (1) and/or ![]()
![]() Topics 10 & 20. Organic chemistry (2)
Topics 10 & 20. Organic chemistry (2)

 IB Docs (2) Team
IB Docs (2) Team