Topics 7 & 17
Including International-mindedness, TOK, and 'Utilization' in Equilibrium
International-mindedness
![]()
![]() 1. The location of Heavy Industries
1. The location of Heavy Industries
It used to be said that the amount of sulfuric acid a country produced was a good measure of the economic strength of the country (see 2. Sulfuric acid – an indicator of the state of the economy in 'Utilization' at the foot of this page). This is because sulfuric acid is the world's largest bulk chemical and is involved in many different industrial processes. However this statement no longer holds true as many rich industrial countries now import much of their sulfuric acid. This is principally due to Health and Safety regulations, environmental targets and the costs of litigation following accidents. Many of the developed countries have stopped building new heavy industry plants and are instead focussing on producing cleaner speciality chemicals using high technology such as bioengineering. The heavy industries now tend to be located more in the less developed countries where the cost of labour is cheaper and the regulations are less stringent.
![]()
![]() 2. The melting of the polar ice caps
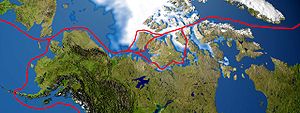
2. The melting of the polar ice caps
Physical systems are also in dynamic equilibrium. These include ice and liquid water (solid-liquid equilibrium) and liquid water and water vapour (liquid-vapour equilibrium). One of the big international concerns is global warming and its effect on the polar ice-caps. In May 2021 a huge iceberg measuring some 170 x 25 km (an area about the size of the Spanish island of Mallorca) broke away from the Antarctic ice cap which threatened marine life in the area. In the northern hemisphere the effect of global warming on the arctic ice has meant that the Northwest Passage shipping route along the northern coast of North America which connects the Arctic and Pacific oceans is now navigable all year round. The Northwest Passage is claimed by Canada as part of their internal territorial waters but other countries claim it is an international shipping route.
Theory of Knowledge
The concept of dynamic equilibrium lends itself to several Theory of Knowledge issues.
![]()
![]() 1. Predictions not explanations
1. Predictions not explanations
When I am marking examination scripts I find that students often confuse predictions with explanations. There are several classic cases where this occurs. One is where students try to explain the product formed by the addition of hydrogen halides to asymmetrical alkenes by simply quoting Markovnikov’s rule. The other example is from equilibrium. Le Chatelier’s Principle enables students to predict what will occur if one of the factors governing the position of equilibrium is altered but it does not explain it. Consider the typical equilibrium reaction quoted for the formation of ethyl ethanoate from ethanol and ethanoic acid:
CH3COOH(l) + C2H5OH(l) ⇄ CH3COOC2H5(l) + H2O(l)
If we remove some of the water from the equilibrium mixture then according to Le Chatelier’s Principle we can predict that more of the ethanoic acid and ethanol will react to try to replace it. I think it is better to get students to be able to explain why as then their prediction will be based on sound deductions rather than just applying a principle. The explanation can be found in the equilibrium expression:
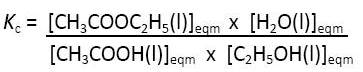
If the concentration of the water is lowered then in order to keep Kc constant more of the ethanoic acid and ethanol must combine to form the ester and water.
![]()
![]() 2. "Seeing is believing"
2. "Seeing is believing"
One of the ways of knowing is to perceive by using the senses. Some years ago one of the ten TOK essay titles effectively asked students to discuss the statement ‘Seeing is believing’. When we look at a system that is in a state of dynamic equilibrium we do not see anything which would lead us to conclude that change is still taking place. For example a gas syringe filled with nitrogen dioxide appears brown. We cannot see that a dynamic equilibrium exists between brown nitrogen dioxide molecules and colourless dinitrogen tetroxide molecules.
2NO2(g) ⇄ N2O4
(brown) (colourless)
If we disturb the equilibrium by pushing the plunger in we will see the mixture momentarily go darker as the concentration of the nitrogen dioxide increases. Then we will see it go lighter than it was initially as the position of equilibrium is restored with a greater concentration of dinitrogen tetroxide present in the equilibrium mixture to counteract the increase in pressure. But once the position of equilibrium is restored the colour remains constant again. So how can we know that the equilibrium really is dynamic and that the rate of conversion of nitrogen dioxide to dinitrogen tetroxide is equal to the rate of the reverse reaction? Fortunately there is a way by using isotopes. Consider a mixture of ice and water at 0 oC. If we make the ice out of heavy water, D2O and surround it by normal water then if the equilibrium is static there will be no D2O present in the liquid water however long we leave it. If the equilibrium is dynamic then although the amount of ice and liquid water will remain constant the liquid water will soon show the presence of D2O. You might like to show your students an interesting video on heavy ice which shows that it is more dense than water and therefore, unlike ice made from normal water, sinks rather than floats.
Heavy water ![]() - a video produced by Nottingham University
- a video produced by Nottingham University
'Utilization'
The Haber Process and the story of Fritz Haber is actually listed under both Theory of Knowledge and International-Mindedness in Topic 7 but in effect it is the utilization of equilibrium.
![]()
![]() 1. The story of Fritz Haber
1. The story of Fritz Haber
The story of Fritz Haber is fascinating. Fritz Haber (1868–1934) was a German chemist who won the Nobel Prize in Chemistry in 1918 for his synthesis of ammonia from nitrogen and hydrogen.
Haber was a complex and highly intelligent man, whose life in many ways provides a good example of the political and social responsibilities facing eminent scientists. His discovery of a method to mIB Docs (2) Teamfacture ammonia from its elements meant that food production could be increased through the use of artificial fertilizers, but his discovery also undoubtedly prolonged the First World War. Ammonia can be oxidized to nitric acid, from which explosives (containing nitrates and nitro compounds) are made. Prior to Haber’s discovery in 1911, nitrates came principally from natural deposits in South America. This meant that the country with the most powerful navy could control the supply of nitrates and hence the production of explosives. Haber’s discovery enabled Germany to become independent of external sources of nitrates.
 During the First World War Haber served his country by using his chemical knowledge to develop gas masks, and also to develop the use of chlorine as the first war gas. Haber himself personally supervised the release of chlorine in the trenches. Wilfred Owen’s poem, “Dulce et Decorum Est”, eloquently refers to the “green sea” of chlorine faced by the soldiers at the time.
During the First World War Haber served his country by using his chemical knowledge to develop gas masks, and also to develop the use of chlorine as the first war gas. Haber himself personally supervised the release of chlorine in the trenches. Wilfred Owen’s poem, “Dulce et Decorum Est”, eloquently refers to the “green sea” of chlorine faced by the soldiers at the time.
.jpg)
Haber’s wife, Clara (right), who was also a chemist, vehemently opposed his developing war gases because she saw them as morally wrong. She took her own life in protest by shooting herself in 1915.
After the war ended, Haber continued to work as a chemist, and helped to develop a substance that produced hydrogen cyanide gas for use as an insecticide. Ironically, this substance, known as Zyklon B, may have been used to kill many of his own relatives, who perished in concentration camps.
Having been honoured by his country for his contribution to science, Haber, who was Jewish, was then forced to emigrate in 1933 to avoid persecution by the Nazis. He died very shortly after this in Switzerland in 1934.
Fritz Haber was a polymath. He was widely read and knowledgeable in history, economics, science and industry. He was an excellent administrator, giving the people who worked for him the freedom to pursue their own ideas, and he was also an excellent communicator. His main discovery had the potential for great good and great evil. His life was one of service to his country, normally a quality that would be praised. Haber defended the use of chemicals in war by stating that death was death, by whatever means it was inflicted. In 1925 the Geneva Protocol banned the use of poison gas in warfare; to date 132 countries have signed this protocol.
You can read more and also listen to the story of Fritz Haber on a BBC programme.
![]()
![]() 2. Sulfuric acid – an indicator of the state of the economy
2. Sulfuric acid – an indicator of the state of the economy
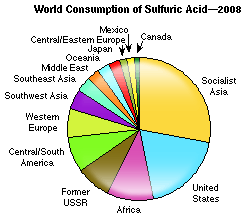 Two very important industrial examples of equilibrium are the Haber process for making ammonia and the Contact Process for making sulfuric acid. Sulfuric acid has many uses. Its main use is in the production of fertilizers. Some of its other uses are in the mIB Docs (2) Teamfacture of dye stuffs, explosives and paper, the pickling and refining of metals and in car batteries. In the mid-19th century Justus von Liebig, a German chemist, commented that the wealth of a nation could be gauged by the amount of sulfuric acid it produced. Nowadays it is more to do with the amount of sulfuric acid used rather than produced as many nations import much of their sulfuric acid rather than produce it themselves. Along with the production of ammonia and sodium hydroxide (caustic soda), it is also a good economic indicator of the state of the economy. According to a report written by Bala Surash for the Chemical Economic Handbook sulfuric acid consumption increased worldwide by about 25% during the period 1990-2008 but with the onset of the ‘credit crunch’ world consumption fell steeply during the second half of 2008, although it has now recovered again.
Two very important industrial examples of equilibrium are the Haber process for making ammonia and the Contact Process for making sulfuric acid. Sulfuric acid has many uses. Its main use is in the production of fertilizers. Some of its other uses are in the mIB Docs (2) Teamfacture of dye stuffs, explosives and paper, the pickling and refining of metals and in car batteries. In the mid-19th century Justus von Liebig, a German chemist, commented that the wealth of a nation could be gauged by the amount of sulfuric acid it produced. Nowadays it is more to do with the amount of sulfuric acid used rather than produced as many nations import much of their sulfuric acid rather than produce it themselves. Along with the production of ammonia and sodium hydroxide (caustic soda), it is also a good economic indicator of the state of the economy. According to a report written by Bala Surash for the Chemical Economic Handbook sulfuric acid consumption increased worldwide by about 25% during the period 1990-2008 but with the onset of the ‘credit crunch’ world consumption fell steeply during the second half of 2008, although it has now recovered again.

 IB Docs (2) Team
IB Docs (2) Team