Topics 6 & 16
Including International-mindedness, TOK, and 'Utilization' in Chemical kinetics
International-mindedness
![]()
![]() 1. Maxwell-Boltzmann curve
1. Maxwell-Boltzmann curve
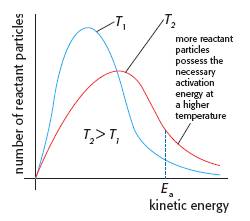 There are many examples in Chemistry where two (or more) people are credited with discovering the same theory or process. For example Watson and Crick for the structure of DNA and Michaelis and Menten for enzyme kinetics. Often the two worked together on the same project and deserve equal recognition. However sometimes the two people worked completely independently in different countries. An example of this is the American Charles Martin Hall and the Frenchman Paul Hérault who are credited with the Hall-Hérault process for the electrolysis of alumina in molten cryolite to produce aluminium. The relevant example for Topic 6 is James Clark Maxwell (1831-1879) a Scottish physicist and mathematician working at Cambridge University and Ludwig Eduard Boltzmann (1844-1906) an Austrian physicist working at the Universities of Graz, Munich and Vienna. Independently they are credited with the distribution curve showing the frequency of velocities or kinetic energies of particles in a liquid or gas. This curve is used to explain how both temperature and catalysts can affect the rate of a chemical reaction.
There are many examples in Chemistry where two (or more) people are credited with discovering the same theory or process. For example Watson and Crick for the structure of DNA and Michaelis and Menten for enzyme kinetics. Often the two worked together on the same project and deserve equal recognition. However sometimes the two people worked completely independently in different countries. An example of this is the American Charles Martin Hall and the Frenchman Paul Hérault who are credited with the Hall-Hérault process for the electrolysis of alumina in molten cryolite to produce aluminium. The relevant example for Topic 6 is James Clark Maxwell (1831-1879) a Scottish physicist and mathematician working at Cambridge University and Ludwig Eduard Boltzmann (1844-1906) an Austrian physicist working at the Universities of Graz, Munich and Vienna. Independently they are credited with the distribution curve showing the frequency of velocities or kinetic energies of particles in a liquid or gas. This curve is used to explain how both temperature and catalysts can affect the rate of a chemical reaction.
One of the biggest grain explosions ever to occur took place at the DeBruce Grain Elevator in Wichita, Kansas, USA in 1998.

One of the factors affecting the rate of a reaction is surface area and explosions of small particles in grain silos and coal dust in coal mines have occurred regularly in many countries around the world.
 Coal dust explosions release huge amounts of energy (much more than methane gas explosions) and can completely destroy mines. One of the worst coal dust explosions ever to occur took place in 1963 at the Miike coal mine in Japan. 1,197 of the 1,403 miners working at the mine were either killed or suffered from severe carbon monoxide poisoning. One of the ways in which the risk of this happening can be minimised is to spread limestone dust around the coal mine so that if there is a small methane (fire damp) explosion the limestone dust rises with the coal dust and lowers the surface area of the exposed coal. Because of the dangers of dust explosions there are many International and European safety standards in operation and many firms (for example, BreGlobal) advertise to carry out tests to these standards.
Coal dust explosions release huge amounts of energy (much more than methane gas explosions) and can completely destroy mines. One of the worst coal dust explosions ever to occur took place in 1963 at the Miike coal mine in Japan. 1,197 of the 1,403 miners working at the mine were either killed or suffered from severe carbon monoxide poisoning. One of the ways in which the risk of this happening can be minimised is to spread limestone dust around the coal mine so that if there is a small methane (fire damp) explosion the limestone dust rises with the coal dust and lowers the surface area of the exposed coal. Because of the dangers of dust explosions there are many International and European safety standards in operation and many firms (for example, BreGlobal) advertise to carry out tests to these standards.
Theory of Knowledge
There is only one reference to TOK on the core part of the programme concerning Chemical kinetics. This asks whether physical properties such as temperature are invented or discovered. The Kelvin scale of temperature gives a natural measure of the kinetic energy of gas whereas the artificial Celsius scale is very much based on the properties of one particular substance, water. There is also a reference to TOK in the AHL. This asks what is the role of empirical evidence in scientific theories and goes on to ask whether we can ever be certain in science. It leads on from the fact that reaction mechanisms can be supported by indirect evidence. It is worth looking at an example to illustrate this point.
![]()
![]() 1. Determining a mechanism
1. Determining a mechanism
Although Chemistry is an experimental science we can often use logic to deduce what is likely to occur when one or more of the variables are altered. However the rate law for a chemical reaction can only be determined experimentally and cannot be deduced theoretically. Once the rate law is established then it is possible to propose a mechanism which is consistent with the experimentally determined rate law but the rate law cannot be used to prove the mechanism (since other possible mechanisms may also produce the same rate expression). However it can be used to disprove a mechanism. This fits in well with Karl Popper’s theory of falsification in science. For example the rate law for the nucleophilic substitution reaction between a tertiary halogenoalkanes and hydroxide ions is found to be first order with respect to the concentration of the halogenoalkanes and is independent of the hydroxide ion concentration. This means that any mechanism that involves the hydroxide ion in the slow step of the reaction cannot be correct.
![]()
![]() 2. Statistical probability
2. Statistical probability
 Rates of reaction can be measured very precisely and one of the ways to determine whether a reaction is first order is to determine whether it has a constant half-life. This raises an interesting philosophical point since individual reacting molecules will have varying amounts of energy and orientation (as shown by the Maxwell-Boltzmann distribution) so how can we be so precise about values for half-lives? Sometimes our very survival depends upon being able to predict the rate of a reaction (such as the time taken for an airbag to inflate after a car accident) and yet we can never predict when an individual molecule will react. Perhaps this is best exemplified by radioactive half-lives. The half-life of 14C is 5730 years and radioactive carbon dating is based on this fact (see my blog on dating teeth). It is however only a statistical probability. If we have just one carbon-14 atom then it is impossible to state when exactly it will break down. It might be in the next second or in a million years' time. This illustrates the importance of statistical probability in Chemistry and underlines how great a quantity one mole is. Even if we only have a very small amount of carbon-14, say 10-6 grams, then we still have in the region of 5 x 1016 atoms – hence our reliance on statistical probability is justified. Some people try to extrapolate this to the human behaviour in the social sciences. As individuals we all behave differently but can we predict how a large number of people will behave with any certainty? The problem is that the number of people involved is always very much smaller than typical numbers of atoms or molecules in a chemical reaction so the prediction will be much less reliable.
Rates of reaction can be measured very precisely and one of the ways to determine whether a reaction is first order is to determine whether it has a constant half-life. This raises an interesting philosophical point since individual reacting molecules will have varying amounts of energy and orientation (as shown by the Maxwell-Boltzmann distribution) so how can we be so precise about values for half-lives? Sometimes our very survival depends upon being able to predict the rate of a reaction (such as the time taken for an airbag to inflate after a car accident) and yet we can never predict when an individual molecule will react. Perhaps this is best exemplified by radioactive half-lives. The half-life of 14C is 5730 years and radioactive carbon dating is based on this fact (see my blog on dating teeth). It is however only a statistical probability. If we have just one carbon-14 atom then it is impossible to state when exactly it will break down. It might be in the next second or in a million years' time. This illustrates the importance of statistical probability in Chemistry and underlines how great a quantity one mole is. Even if we only have a very small amount of carbon-14, say 10-6 grams, then we still have in the region of 5 x 1016 atoms – hence our reliance on statistical probability is justified. Some people try to extrapolate this to the human behaviour in the social sciences. As individuals we all behave differently but can we predict how a large number of people will behave with any certainty? The problem is that the number of people involved is always very much smaller than typical numbers of atoms or molecules in a chemical reaction so the prediction will be much less reliable.
'Utilization'
![]()
![]() 1. Economic importance
1. Economic importance
The ultimate aim of all industries is to make money. Understanding how a reaction works can often provide a cheaper and more efficient route which will give an industry an edge over its competitors. One of the ways in which this can be achieved is to develop more effective (and perhaps cheaper) catalysts. For example, one of the factors that increases the costs of automobiles is the use of precious metals such as rhodium, platinum or palladium in catalytic convertors in the exhaust system. Much research is taking place to find cheaper alternatives and to date cerium, iron, manganese and copper have all been used with varying degrees of success[1].
![]()
![]() 2. The environment – effect of water temperature on fish life
2. The environment – effect of water temperature on fish life
 The rate of metabolism in fish increases as the temperature increases and so they will require more oxygen to survive in warmer water. One of the ways in which water gets polluted is through thermal pollution when industries release waste hot water (that has usually been used for cooling) into nearby waterways. Oxygen is not particularly soluble in water (and even less soluble in salty water) and the dissolving is an exothermic process. By applying the equilibrium law (Le Chatelier’s Principle) students can see that the solubility of oxygen in water will decrease as the temperature of the water increases. (A useful table of how temperature and salinity affects the solubility of oxygen in water can be downloaded from Insite Instrumentation Group Incorporated
The rate of metabolism in fish increases as the temperature increases and so they will require more oxygen to survive in warmer water. One of the ways in which water gets polluted is through thermal pollution when industries release waste hot water (that has usually been used for cooling) into nearby waterways. Oxygen is not particularly soluble in water (and even less soluble in salty water) and the dissolving is an exothermic process. By applying the equilibrium law (Le Chatelier’s Principle) students can see that the solubility of oxygen in water will decrease as the temperature of the water increases. (A useful table of how temperature and salinity affects the solubility of oxygen in water can be downloaded from Insite Instrumentation Group Incorporated ![]() .) Fish therefore suffer in warm water by requiring more oxygen to metabolise and there is even less oxygen present than at lower temperatures. Sadly this too often leads to headlines such as ‘Thousands of fish die from heat’.
.) Fish therefore suffer in warm water by requiring more oxygen to metabolise and there is even less oxygen present than at lower temperatures. Sadly this too often leads to headlines such as ‘Thousands of fish die from heat’.
Footnotes
- ^ The choice of catalyst provides an interesting example of International-mindedness as it is illegal to use nickel as the catalyst in catalytic convertors in the European Union (as it can react with carbon monoxide to form nickel carbonyl) whereas it is illegal to use copper in the United States of America (as it can react to form dioxins) but it can be used elsewhere.

 IB Docs (2) Team
IB Docs (2) Team