A.5 Polymers
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Option A - sub topic A.5. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.
.png)

 Learning outcomes
Learning outcomes
After studying this topic students you be able to:
.png) Understand:
Understand:
- Thermoplastics harden when cooled and soften when heated.
- Thermosetting polymers are prepolymers in a soft solid or viscous state, which irreversibly change into a hardened thermoset by curing.
- Elastomers are flexible. They can be deformed under force but will effectively return to their original shape once the stress is removed.
- Because high density poly(ethene) (HDPE) has no branching the chains are packed closely together.
- Low density poly(ethene) (LDPE) is more flexible as it has some branching.
- The flexibility of a polymer can be increased by adding plasticizers as they weaken the intermolecular forces between the polymer chains.
- The atom economy is a measure of efficiency applied in green chemistry.
- In isotactic addition polymers the substituents are on the same side whereas in atactic addition polymers the substituents are randomly situated.
Apply your knowledge to:
- Describe the use of plasticizers in polyvinyl chloride and the use of volatile hydrocarbons in the formation of expanded polystyrene.
- Solve problems and evaluate the atom economy in synthesis reactions.
- Describe how the properties of polymers depend upon their structural features.
- Describe ways of modifying the properties of polymers, including LDPE and HDPE.
- Deduce the structures of polymers formed from the polymerization of 2-methylpropene.
Relationships & vocabulary
Nature of science
Due to advances in technology (e.g. X-ray diffraction, scanning tunnelling electron microscopy, etc.), scientists are able to better understand polymerisation at the molecular level. This enables matter to be manipulated in new ways leading to the development of new polymers.
Staudinger's theory that macromolecules are comprised of many repeating units was integral in the development of polymer science.
The rapid development and use of polymers has increased faster than an understanding of the risks involved (e.g. recycling or possible carcinogenic properties).
International-mindedness
Before the second world war plastics were virtually unknown. Consider how the introduction of plastics has affected the world in economic, social and environmental terms.
Vocabulary
| thermoplastic (or thermosoftening) | thermosetting | prepolymer |
| high density poly(ethene) (HDPE) | elastomer | isotactic |
| low density poly(ethene) (LDPE) | plasticizer | atactic |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
The syllabus for this sub-topic covers the use of plasticizers to make PVC flexible but it does not actually cover what they are. Probably the most commonly used plasticizers are the phthalates which are mentioned in sub-topic A.7 Environmental impact - plastics. Technically phthalate plasticizers are dialkyl or alky-aryl esters of phthalic acid, 1,2-benzenedicarboxylic acid.
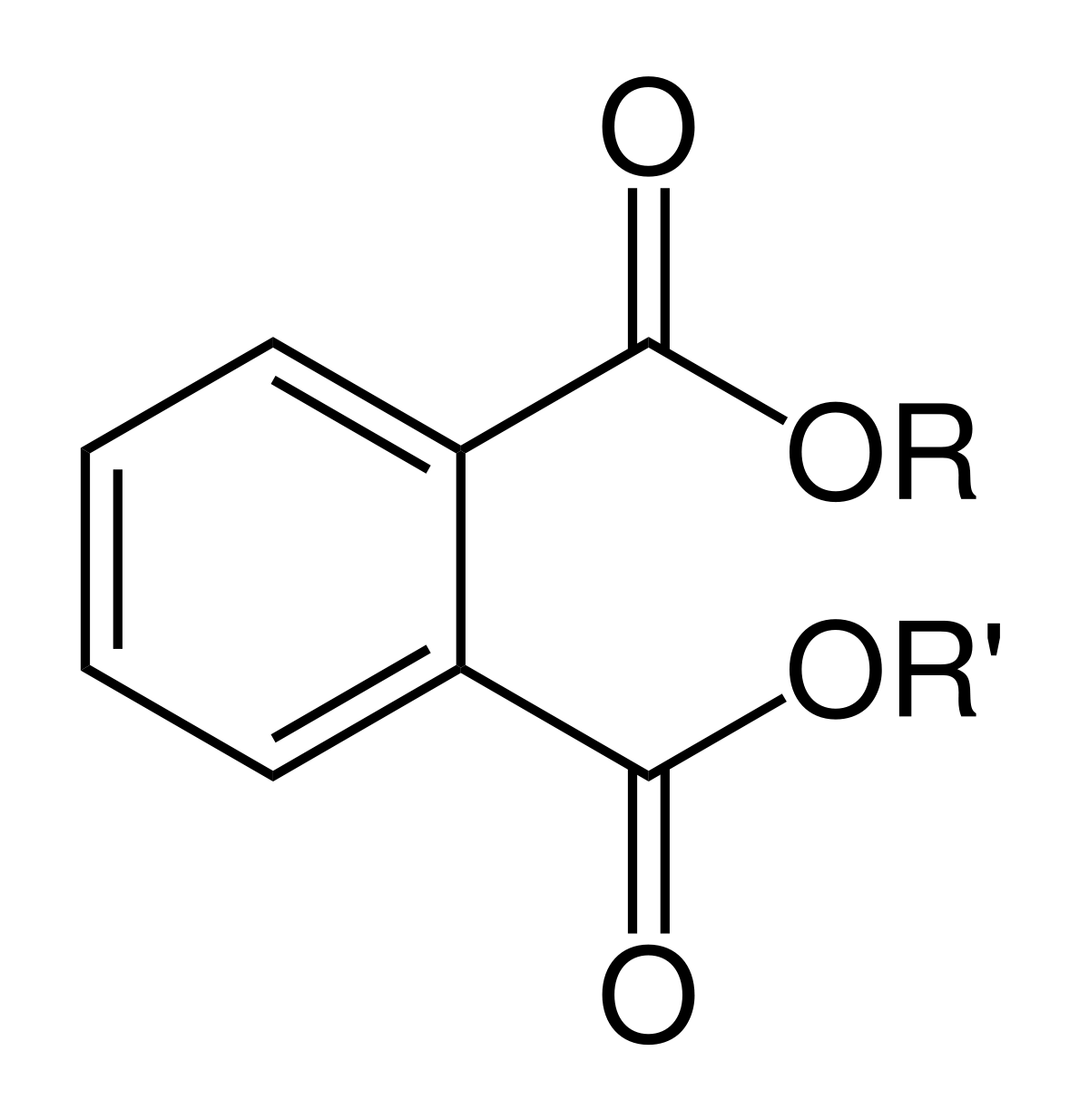
General formula for phthalate esters
They are commonly divided into two classes depending upon the molar masses of the two R- groups. Low molecular mass phthalates contain three to six carbon atoms in their R- group side chains and high molecular mass phthalates contains more than six carbon atoms in their side chains.
An example of a low molecular mass phthalate is dimethyl phthalate (DMP), R = R’ = − CH3 where Mr = 194.2, and an example of high molecular mass phthalate is di(n-octyl) phthalate (DNOP), R = R’ = −(CH2)7CH3 where Mr = 390.6.
The distinction is important because the use of low molecular mass phthalates is being phased out, particularly in North America and Europe, over concerns about safety issues. The low molecular mass phthalates are more volatile and their presence can commonly be detected in the urine of humans. Although it has been claimed that phthalates are carcinogenic there appears to be no absolute hard evidence to support this. They have also been linked to increases in asthma. In many countries their use is either completely banned or heavily restricted in certain children’s (and adults’!) toys to lower the possibility of ingestion.
Phthalates work as plasticizers in PVC by forming polar interactions between the polar δ− oxygen atoms on the C=O groups on the phthalate molecules and the δ+ carbon atoms on the vinyl chain due to the C-Cl bond. For this to happen the polymer needs to be heated in the presence of the plasticizer and the interactions then remain when the polymer is cooled thus weakening the attractions between the polymer chains and making the polymer more flexible.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Polymers.
For short-answer questions see Polymers questions together with the worked answers on a separate page Polymers answers.
More resources
1. A rather nice way to see how far we have come in the past sixty years. An unashamedly propaganda film made by B.F. Goodrich in 1954 on man-made rubber.
2. A very simple explanation of how alkene monomers form addition polymers by BBLC.mov.
3. Plasticisers and Hardeners - a straightforward and clearly illustrated video by Fuseschool.

 IB Docs (2) Team
IB Docs (2) Team 














