A.10 Environmental impact - heavy metals
Written specifically for students to provide help and support for the IB Diploma chemistry programme this page provides full coverage of the syllabus content of Option A - sub topic A.10. It encourages you to think critically and provides many questions with full worked answers so that you can monitor and improve your knowledge and understanding.

 Learning outcomes
Learning outcomes
After studying this topic you should be able to:
 Understand:
Understand:
- The normal oxidation/reduction balance in cells can be disturbed by toxic doses of transition metals through various mechanisms.
- Methods of removing heavy metals include precipitation, adsorption, and chelation.
- Due to the chelate effect polydentate ligands tend to give more stable complexes than monodentate
ligands. This can be explained by considering entropy changes.
Apply your knowledge to:
- Explain the use of chelating substances to remove heavy metals.
- Deduce the number of coordinate bonds a ligand can form with a central metal ion.
- Solve problems involving Ksp as an application of removing metals in solution.
- Compare and contrast the Fenton and Haber–Weiss reaction mechanisms.
Relationships & vocabulary
Nature of science
Although scientific research often proceeds with perceived benefits in mind, there is a responsibility to also consider the risks and implications.
International-mindedness
Consider the responsibilities that scientists have for the global impact of their work.
Vocabulary
| chelate/chelation | sequestration | coordination number | polydentate ligand |
| ethane−1,2−diamine | ethylenediaminetetraacetic acid, EDTA | solubility product, Ksp |
Learning slides
You can use this slide gallery for learning or for reviewing concepts and information. It covers all the key points in the syllabus for this sub-topic.
Something to think about
You will need will to take some care when using solubility products.
Consider the following values (at 298 K) which are obtained from the table given in Section 32 of the data book1.
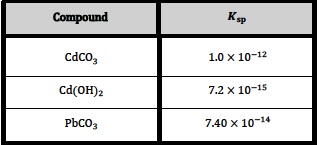
An obvious question might be; Which one of the above salts has the lowest solubility in water at 298 K?
Looking at the values for solubility products the answer might appear to be cadmium(II) hydroxide as its Ksp value is considerably lower than the Ksp values for the other two salts. In earlier IB programmes students were expected to know that equilibrium constants had units and they could be asked to deduce them for particular reactions. Since then the IB has correctly stated that equilibrium constants have no units but this can lead to a little confusion when faced with a table of values such as the one given above. To compare the values meaningfully, you need to consider what equilibrium system each Ksp value is referring to.
Consider the first value of 1.0 x 10−12 for cadmium carbonate. This refers to:
CdCO3(s) ⇌ Cd2+(aq) + CO32−(aq)
and Ksp = [Cd2+(aq)] x [ CO32−(aq)]
For a saturated solution of cadmium(II) carbonate at 298 K, [Cd2+(aq)] = [ CO32−(aq)] = (1.0 x 10−12)½ = 1.0 x 10−6 mol dm−3
so the solubility of cadmium(II) carbonate at 298 K is 1.0 x 10−6 mol dm−3.
For cadmium(II) hydroxide, Ksp at 298 K is 7.2 x 10−15. The equilibrium system this value refers to is:
Cd(OH)2(s) ⇌ Cd2+(aq) + 2OH−(aq)
and Ksp = [Cd2+(aq)] x [OH−(aq)]2
For a saturated solution of cadmium(II) hydroxide [OH−(aq)] = 2 x [Cd2+(aq)]
so Ksp = 4 x [Cd2+(aq)]3, which means [Cd2+(aq)] = (7.2 x 10−15 ÷ 4)⅓ = (1.8 x 10−15)⅓ = 1.2 x 10−5 mol dm−3
The solubility of cadmium(II) hydroxide in water at 298 K is therefore 1.2 x 10−5 mol dm-3 which means that it is more soluble in water than cadmium(II) carbonate at 298 K.
However the Ksp value for lead(II) carbonate at 298 K is 7.40 x 10−14. By carrying out a similar calculation to the ones above it can be seen that the solubility of lead(II) carbonate in water at 298 K is 2.7 x 10−7 mol dm−3. Thus lead(II) carbonate is the least soluble of the three salts.
Although it would not affect the order for these three salts, note too that if solubilities are expressed in g dm−3 rather than mol dm−3 then this may also affect the order if the molar solubilities are close in value.
Footnote
1Note that some of the Ksp values in the data booklet are given to two significant figures and some are given to three significant figures.
Test your understanding of this topic
(Note that your teacher may have restricted your access to some or all of these questions and worked answers if they are going to use them as a class test or set them as an assignment.)
For ten 'quiz' questions (for quick testing of knowledge and understanding with the answers explained) see MC test: Environmental impact - heavy metals.
For short-answer questions see Environmental impact - heavy metals questions together with the worked answers on a separate page Environmental impact - heavy metals answers.
More resources
1. A very simple explanation of Ksp using lead(II) iodide as an example by chemistNATE.
2. An animation of the common ion effect using silver iodide as an example.
3. A (non-IB) student demonstrates her Science Fair project on Fenton's reagent.
![]() Demonstration of Fenton's reagent.
Demonstration of Fenton's reagent.

 IB Docs (2) Team
IB Docs (2) Team 




















