Topics 5 & 15
Including International-mindedness, TOK, and 'Utilization' in Energetics/thermochemistry
International-mindedness
Energy production and consumption are worldwide issues. There are many reasons why wars start. Often it is over territorial claims (for example the Second World War and the Russian invasion of Ukraine in 2022 ) but some have claimed that the real reason for the two Gulf Wars of 1991 and 2003 was to protect oil routes and interests. Other International-Mindedness connections include:
![]()
![]() 1. Oil production
1. Oil production
The recent problems over the huge oil spill in the Gulf of Mexico highlight the multinational aspect of oil research and production. I have talked about this on my blog entry Chemistry and politics. The oil spill occurred in USA territorial waters by BP, a company with strong British connections on the Deepwater Horizon oil platform which was built in S Korea and is owned by Transocean, a company with offices in more than 30 countries.
![]() 2. The Second Law of Thermodynamics
2. The Second Law of Thermodynamics
Perhaps the ultimate example for International-Mindedness is the application of the Second Law of Thermodynamics to the whole universe. Although the Law as such is not on the syllabus it is covered in reality in 15.2 Entropy & spontaneity as it involves entropy. In simple terms it states that heat cannot pass from a colder body to a hotter body but another way of stating it is that the entropy of an isolated system not at equilibrium will tend to increase over time approaching a maximum value. Some people conjecture that the Second law of Thermodynamics predicts the eventual end of the universe (assuming that the universe is an isolated system).
Theory of Knowledge
The syllabus is rather bare when it comes to suggesting connections between Energetics/thermochemistry and TOK, In fact there are only three references to TOK. Sub-topic 5.1 Measuring energy changes asks "What criteria do we use in judging discrepancies between experimental and theoretical values?" and "Which ways of knowing do we use when assessing experimental limitations and theoretical assumptions?". Sub-topic 5.2 Hess's Law asks "What are the challenges and limitations of applying general principles to specific instances? and Sub-topic 15.2 Entropy & spontaneity states "Entropy is a technical term which has a precise meaning. How important are such technical terms in different areas of knowledge?" This last statement could equally well be extended to 'spontaneous' as this word has a completely different meaning in chemistry to its everyday use in English. When you teach Hess's Law it is well worth explaining to your students that this is simply another way of expressing the First Law of Thermodynamics, in other words the Law of Conservation of Energy. If Hess's Law was not true then you could use a chemical reaction to produce an unlimited amount of energy - and solve many of the problems of the world!
![]()
![]() 1. Assumptions about enthalpy, H and standard enthalpy changes, ΔH
1. Assumptions about enthalpy, H and standard enthalpy changes, ΔH![]()
Perhaps a better connection with TOK is to look at some of the underlying assumptions we make when teaching Energetics/thermochemistry. Students will finish the course with an understanding of enthalpy and standard enthalpy change of reaction using the symbols H and ΔH![]() respectively. In fact these are rather simplified definitions. H is the enthalpy content of a substance but it cannot be measured. Even so, one of the first things we show students is an enthalpy level diagram where one of the axes is enthalpy. We tell students that graphs should have labelled axes and the values should be given together with the units but if we cannot measure enthalpy then how can we place the values of the initial and starting states of the reaction on the diagram?
respectively. In fact these are rather simplified definitions. H is the enthalpy content of a substance but it cannot be measured. Even so, one of the first things we show students is an enthalpy level diagram where one of the axes is enthalpy. We tell students that graphs should have labelled axes and the values should be given together with the units but if we cannot measure enthalpy then how can we place the values of the initial and starting states of the reaction on the diagram?
Of course the difference between the two values can be measured but that does not mean that the graphs are correct. Perhaps it would be better to label the y axis as relative enthalpy value?
Students may also get the impression that the enthalpy change is always given by the expression ΔH⦵ but this is only true if the enthalpy change is measured at constant pressure. For a gaseous reaction where there is a volume change then work will be done in changing the volume (imagine pushing a piston in or out as the volume of a cylindrical container of the gas changes) in order to maintain a constant pressure. If the enthalpy change is measured at constant volume then the value will be different as no external work is done. This value is known as ΔU⦵ and the relationship between ΔH⦵ and ΔU⦵ is given by the expression ΔH⦵ = ΔU⦵ + Δ(pV) where Δ(pV) is equivalent to the work done.
Finally an interesting TOK point arises when we use enthalpy changes for non-existent reactions in order to gain useful information about something else. Perhaps this is the mathematical equivalent of using complex numbers (such as the square root of minus one) to solve problems? The classic example of this is finding the value of ΔH⦵ for the formation of methane from its elements (carbon and hydrogen do not react to give only pure methane under any conditions) and then using the value to determine the C–H bond enthalpy.
Applying critical thinking to the Born-Haber cycle raises several interesting points. When I set an IB exam question recently which included a diagram of a Born-Haber cycle several teachers complained that they did not recognize the format.
Many books and many teachers do not actually use a cycle they use an enthalpy level diagram (see left) which is strictly not a Born-Haber cycle.
I think this can be quite confusing to students as they need to know beforehand whether individual steps are exothermic or endothermic which is not always obvious when it comes to electron affinities (chlorine is exothermic but oxygen is endothermic).
.gif) I think it is better to train students to actually use a cycle (see right) as the Born-Haber cycle is simply a special case of a general energy cycle. I accept that this is not of major importance but since the syllabus ( 15.1 Energy cycles) actually states Born-Haber cycle it seems odd that some students and (teachers) do not recognize it when it is set out correctly as a cycle rather than as an enthalpy diagram.
I think it is better to train students to actually use a cycle (see right) as the Born-Haber cycle is simply a special case of a general energy cycle. I accept that this is not of major importance but since the syllabus ( 15.1 Energy cycles) actually states Born-Haber cycle it seems odd that some students and (teachers) do not recognize it when it is set out correctly as a cycle rather than as an enthalpy diagram.
However the main point arises when comparing the lattice enthalpy values obtained theoretically and experimentally from the cycle. Normally it is taken that the theoretical value is based on a purely ionic model (using an equation considering point charges in the crystal developed by Erwin Madelung) and one looks to see whether there is good agreement with the experimental value obtained from the Born-Haber cycle. If there is (e.g. with sodium chloride) then the compound is said to be close to 100% ionic in character whereas if they differ (e.g. for silver iodide) one assumes the compound has an appreciable amount of covalent character. But if you examine the cycle closely it too is based on a purely ionic model so which one is the correct true value?
'Utilization'
There are very obvious connections between Energetics/thermochemistry and 'Utilization' as the combustion of fuels poses many ethical, economic and environmental issues. Questions you can ask students include:
- Should we be burning all our oil, or should we be conserving it to make other products such as plastics and medicines?
- Should we accept human rights abuse in countries that have large reserves of oil as we do not wish to offend them by being critical?
- Should we be promoting electric cars when the conversion of energy to electricity is one of the least efficient conversions?
- Should agricultural land be used for growing crops for conversion to biofuels rather than food for consumption?

- Is it really valid to offset our carbon footprint every time we fly or is it just a sop to our conscience?

 IB Docs (2) Team
IB Docs (2) Team 
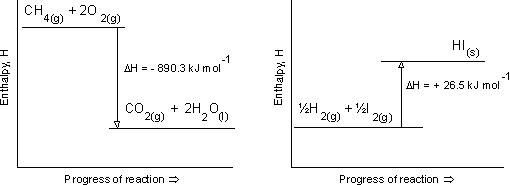
.jpg)