Topics 3 & 13
Including International-mindedness, TOK, and 'Utilization' in Periodicity and Transition elements
International-mindedness
![]()
![]() 1. The universal periodic table
1. The universal periodic table
The obvious example of an international dimension for periodicity is the periodic table itself. Even if it is written in a completely different script to one that you can read it is still obvious which element is which due to its position in the periodic table as the table itself is recognisable in one of its usual formats. In fact the universality of the language of chemistry is more than this as the symbols of the elements are the same whatever the language. For example, the ones below are in Arabic and Mandarin.
![]()
![]() 2. The language of chemistry
2. The language of chemistry

Look at a section from the book in the picture below.
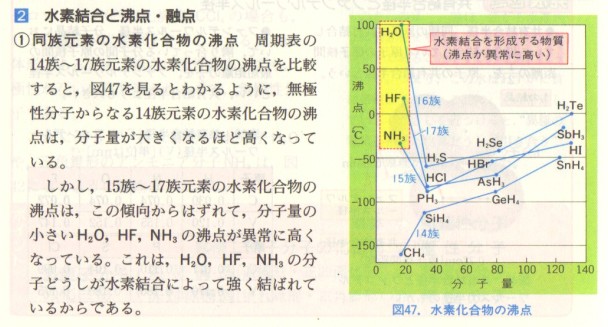
Even without knowing any Japanese it is obvious that the section is explaining that the anomalous boiling points of water, ammonia and hydrogen fluoride compared to the rest of the hydrides in their respective groups are due to hydrogen bonding. Chemists need to be able to communicate with each other internationally and much, but not all[1], of the language of chemistry (and the mathematics it uses) is universal. Because of its importance there is a separate page on this website on the Language of Chemistry in the Theory of Knowledge section.
![]()
![]() 3. Discovery of the elements
3. Discovery of the elements
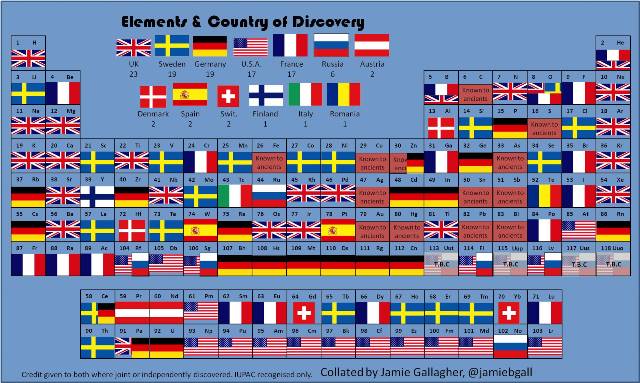
One rather neat way to show how scientists from many different countries have contributed to chemistry is to portray the periodic table by country of discovery. The following table, put together by Jamie Gallagher, is from Smithsonian.com.
Theory of Knowledge
![]()
![]() 1. Emotion and risk taking in gaining knowledge
1. Emotion and risk taking in gaining knowledge
The proposal of a periodic table by Mendeleev in 1869 must rank as one of the greatest achievements in science. Mendeleev could not logically deduce a relationship between the elements from the elements known at the time. He had to use his imagination and take a leap of faith (i.e. be a risk taker) that some elements still remained to be discovered if his periodic table was to work. Science is about making predictions and Mendeleev's prediction for the chemical and physical properties for the as then undiscovered element eka-silicon (now known as germanium) was truly amazing in its accuracy.
![]()
![]()
![]() 2. The paradigm of the Periodic Table
2. The paradigm of the Periodic Table
The periodic table is so fundamental to chemistry that it is taken as one of the basic cornerstones of the subject and is rarely questioned. It is a fantastic achievement and clearly provides an almost unchallenged paradigm for chemists. Graphs like the 1st. ionization energy against atomic number (see the section on this which is discussed under Areas of difficulty with paper 2) show almost perfect periodicity, although graphs of some of the other properties against atomic number are slightly less convincing. Some trends do not continue across the period or down a group and can be explained. For example, the formulas for the highest fluorides of the elements of period 3 goes NaF, MgF2, AlF3, CF4, PF5, SF6 but then comes ClF5 not ClF7. This is because there is simply not room enough to accommodate seven fluorine atoms around a chlorine atom. Make the halogen bigger and the space will be available and iodine heptafluoride, IF7, does exist. However some trends do not fit and the explanation is not so forthcoming. The electron affinities of the halogens do not decrease regularly down the group as chlorine has a higher exothermic value than fluorine:
Table showing the electron affinities of the halogens
| Element | Electron affinity / kJ mol-1 |
| Fluorine, F | – 328 |
| Chlorine, Cl | – 349 |
| Bromine, Br | – 325 |
| Iodine, I | – 295 |
Perhaps one day a more modern paradigm of periodicity will explain all that the periodic table currently explains and also the anomalies as well. We are so steeped in the periodic table that it is hard to see this ever happening – but then that is exactly what has happened in history when other paradigm shifts have occurred.
'Utilization'
![]()
![]() 1. Pollution due to acidic non-metal oxides
1. Pollution due to acidic non-metal oxides
Acid rain which causes considerable damage to crops, building and aquatic and marine life is caused by the increase in two major pollutant gases. Sulfur dioxide from the combustion of fossil fuels, particularly coal, in heavy industry and oxides of nitrogen, NOx, which are formed when nitrogen and oxygen combine at the high temperatures that occur inside an internal combustion engine. In the air these form sulfuric acid and nitric acid with a pH less than 5.6. Rain naturally contains dissolved carbon dioxide which can reduce the pH to 5.6 hence acid rain is defined as rain with a pH less than 5.6. There is much more about acid deposition in Topic 8.5.
![]() 2. Pollution due to aluminium ions
2. Pollution due to aluminium ions
In the first editions of the 'Guidance' section of Topic 18.3 it stated "The acidity of hydrated transition metal ions is covered in topic 13. The treatment of other hydrated metal ions is not required." - even though there seemed to be nothing actually on the acidity of hydrated transition metal complex ions in Topic 13. This misleading statement has subsequently been removed but was probably there because in the previous programme the acidity of iron(III) chloride and aluminium (III) chloride was on the syllabus and Higher Level students needed to know that apart from sodium, the chlorides of the period 3 elements are acidic. For aluminium this is explained by the fact that the aluminium ion, Al3+, has an extremely small radius and therefore the charge density of the ion is extremely high. An Al3+ ion in water will form a coordinate bond with one of the lone pairs on the oxygen atom of a water molecule forming an –OH group bonded to the aluminium and releasing a proton. Thus a solution of any salt of aluminium is acidic. Aluminium ions are used to help flocculation during the treatment of sewage water. There are strict guidelines on the safe limit of aluminium ions in drinking water. In the UK in 1988 a lorry driver inadvertently poured 20 tonnes of aluminium sulfate into water that was then distributed directly to a town called Camelford. The resulting pollution caused many problems such as gastric and urinary complaints as well as skin rashes and arthritic pains. In addition thousands of fish died. Later it has been linked with an increase in the incidence of Alzheimer’s disease in the area. As well as the aluminium ions, the high acidity of the water also increased the concentration of other metal ions, such as lead(II), Pb2+(aq), and zinc(II), Zn2+(aq), from pipes etc.
The ethical issues of adding any chemicals to public water supplies (e.g. fluoride to protect teeth) can also be discussed.
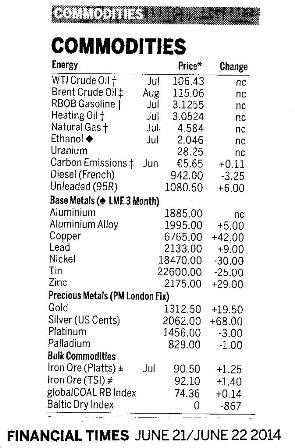
![]() 3. Economic importance of catalysts
3. Economic importance of catalysts
The Haber and Contact processes are important examples of the economic importance of catalysts but there are many more examples. In general, industry is not philanthropic – it exists to make money. Catalysts not only speed up the rate of a chemical reaction they make it more economical as more products can be formed using less energy in less time.
You can find out if the world is entering or coming out of a recession by reading newspapers – not the articles but the trends in the price of commodities (see right). The price of precious metals such as platinum and palladium does not depend on the amount of jewellery being bought - it depends on the amount of vehicles being mIB Docs (2) Teamfactured and sold as these metals are needed to make catalytic convertors.
Footnotes
- ^ For an example of how the language of Chemistry can differ in different cultures look at the section on 'The octet rule versus expanding the octet' in Topics 4 & 14.

 IB Docs (2) Team
IB Docs (2) Team