The Nature of Science
What is the Nature of Science?
There are essentially five distinct points that you should focus on regarding the Nature of Science and in particular how it applies to chemistry.1. What is science and what is the scientific endeavour?
2. The understanding of science
3. The objectivity of science
4. The human face of science
5. Scientific literacy and the public understanding of science
There is clearly much overlap with the Theory of Knowledge course, particularly with the Areas of Knowledge (AOK) that covers the Natural Sciences and it appears to be generally agreed that the Nature of Science is essentially the application of TOK to the Natural Sciences.
I asked one of TOK senior examiners to compare the Nature of Science as written in the chemistry subject guide with the TOK guide. He relied rather eloquently:
"What is written in chemistry guide under Nature of Science are areas that could/would be looked at in TOK in a specific sense, whereas what is written in the TOK guide is very generic. Pretty much everything under the nature of science in the chemistry guide could be explored from a TOK perspective, but the way it is looked at would probably be formulated into questions. For example, in the chemistry guide it states:
"Scientists must adopt a skeptical attitude to claims. This does not mean that they disbelieve everything, but rather that they suspend judgment until they have a good reason to believe a claim to be true or false. Such reasons are based on evidence and argument."
In a TOK sense this might be set out as the question: "To what extent does doubt play a role in the acquisition of knowledge in the natural sciences and how does this effect reliability and validity in this subject area?"
Similarly the chemistry guide gives the statement:
"Evidence is used to develop theories, generalize from data to form laws and propose hypotheses. These theories and hypotheses are used to make predictions that can be tested. In this way theories can be supported or opposed and can be modified or replaced by new theories."
In TOK this might become: "Why is it important to distinguish between hypotheses, laws and theories in the natural sciences and what do these distinctions tells us about knowledge in these areas?" I would suggest that from a student's perspective there is no difference between the two sections. If you were to re-label "nature of science" as "nature of scientific knowledge" then, in my view, this would be a one sentence description of what we do in TOK when we look at the natural sciences."
Another, an author of a highly respected book on TOK, simply said,
“In my book, this is TOK! If not, I would like someone to explain the difference. In one sense, I don’t see any problem with this. TOK is supposed to complement the focus on methodology & critical thinking that should naturally happen in subject areas. Historians talk about primary and secondary sources and we do that in TOK too. However, I think it would create a lot of confusion if the Nature of Science and the Science element of TOK are quite different.
Where there is confusion is that NOS can be assessed whereas TOK will not be assessed in chemistry. The problem with this is that there is considerable overlap between what is written for NOS (assessable) and TOK (not-assessable). To give one example, in 1.1 under TOK it states “Lavoisier’s discovery of oxygen, which overturned the phlogiston theory of combustion, is an example of a paradigm shift. How does scientific knowledge progress?” and yet in Topic 2.1 under NOS it states, “Paradigm shifts—the subatomic particle theory of matter represents a paradigm shift in science that occurred in the late 1800s.” From this it seems quite clear that the concept of paradigm shifts can be examined. (Note that in the updated version of the Guide published in February 2018 the statement 'Lavoisier’s discovery of oxygen' has now been changed to just 'The discovery of oxygen' as Lavoisier did not actually discover oxygen - he only realised its significance).

.jpg)
The human face of science
Einstein’s paradigm shift is NOS so can be assessed; Lavoisier’s paradigm shift is TOK so cannot be assessed!!!!
So what is the best way to incorporate NOS into your learning?
My suggestions are:
- Make sure you understand how TOK works and how it can be applied to chemistry. You can read about this in the TOK section. You can then use specific examples in chemistry when writing your TOK essay or in your exhibition. This is because TOK examiners tend to give credit for relevant specific examples taken from subjects studied by students to back up their arguments. Too often they just see students using the typical examples provided by TOK class teachers who of course cannot be experts in all the different IB disciplines. There are many good examples of TOK/NOS as it relates to chemistry in Peter Wother's book Antimony, Gold & Jupiter's Wolf which I've reviewed in How the elements were named.
- Look at what I have written to address the NOS content on the relevant page for each sub-topic in Full coverage of each topic and sub-topic. Often what is written under 'Something to think about' for each sub-topic relates to the Nature of Science. Also look at what is written for each topic under the heading “Incorporating IM, TOK, Utilization etc.”.
- Use the accompanying glossary of Key terms & concepts. If you know what is meant by key terms such as ‘Paradigm shift’ and Occam’s razor’ and can illustrate them with examples then you will be well-prepared to answer NOS questions.
- Practice with past NOS questions and with the Nature of Science questions linked to this page. Certain areas of NOS such as falsification and the need for repeatable and accurate data will crop up time and time again.
A resource to get you thinking
As an introduction to the Nature of Science you might like to look at the 14 minute TEDx talk given by a theoretical physicist, Dr Teman Cooke, entitled “The scientific method is crap”.
In this talk he defines what he sees as the scientific method as being linear in nature that follows the sequence
Step 1. Identify a problem
Step 2. Do some research
Step 3. Form a hypothesis
Step 4. Do an experiment with independent and dependent variables to test your hypothesis
Step 5. Analyse your data
Step 6. Draw a conclusion
You may well recognise this as a basic formula used by many students for both their IA and EE in chemistry.
He then goes on to talk about the ‘Cycle of scientific thinking’.
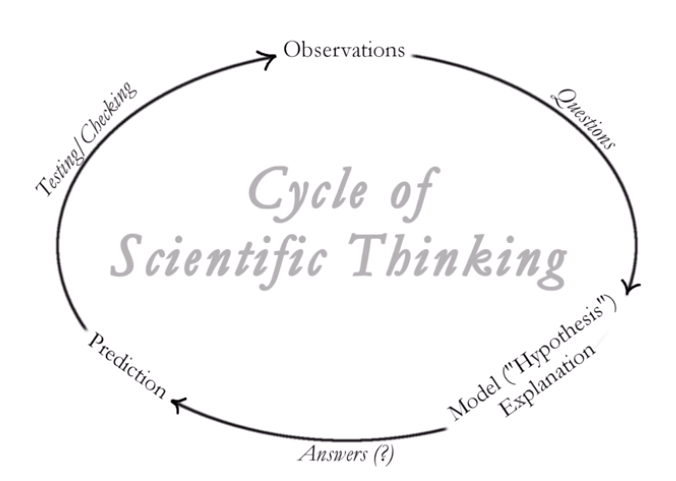
To get you thinking and questioning you might like to reflect on to some of the comments posted below the YouTube video, For example:




 IB Docs (2) Team
IB Docs (2) Team