 Assignment:
Assignment:  Questions on Topic 16.2: Activation energy
Questions on Topic 16.2: Activation energy
This page of questions can be marked as direct student access either for assigning as a test or for students to work on in their own time. If you do not wish to use student access, links to downloadable versions of the questions and, separately the worked answers, can be found at Printable versions of written tasks.
At a temperature of 700 K the rate constant for the redox reaction between nitrogen(IV) oxide and carbon monoxide is 1.30 mol−1 dm3 s−1.
NO2(g) + CO(g)→ NO(g) + CO2(g) k = 1.30 mol−1 dm3 s−1 at 700 K
i. Deduce the overall order of this reaction.
1 line
Since the units of the rate constant are mol−1 dm3 s−1 the overall order of the reaction must be two (i.e. second order).
ii. The activation energy is 133.8 kJ mol−1.
Determine the temperature when the rate constant for the reaction will be 20.0 mol−1 dm3 s−1.
10 lines
ln
k = −
Ea/
RT + ln
AAt 700 K: ln 1.30 = – (133.8 x 1000)/(8.314 x 700) + ln
AHence ln
A = ln 1.30 + (133.8 x 1000)/(8.314 x 700)
At T, ln 20.0 = – (133.8 x 1000)/(8.314 x T) + ln A
ln 20.0 = – (133.8 x 1000)/(8.314 x T) + ln 1.30 + (133.8 x 1000)/(8.314 x 700)
Hence ln 20.0 − ln 1.30 = (133.8 x 1000)/(8.314) x (1/700 − 1/T)
2.7334 = 22.990 −16093/T
T = 16093/20.257 = 794 K (521 oC)
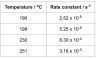
In the gas phase methyl isocyanide, H3CNC, rearranges to form ethanenitrile, H3CCN.

The table below shows the experimentally determined values of the rate constant for this rearrangement at different temperatures.

i. Determine the overall order of the reaction.
1 line
Since the units of the rate constant are s−1 the overall order of the reaction must be one (i.e. first order).
ii. Write the rate expression for the reaction.
1 line
Rate = k[H3CNC]
iii. Using a graphical method, determine the activation energy for the rearrangement of methyl isocyanide.
10 lines
The temperatures need to be converted into Kelvin then a graph of ln
k against 1/
T must be plotted. The gradient is equal to –
Ea/
R.

The gradient = − 4.2/0.00022 = –19091 = – Ea/R
Ea = 19091 x 8.314 = 159000 J = 159 kJ mol−1
(Note that if the value for A was required the ln k axis would need to be extended so the line could be extrapolated to give the value of ln k when 1/T = zero. Alternatively the value for Ea can be put in the equation ln k = – Ea/RT + ln A for one of the values of T and A can be calculated directly.)
iv. Determine the value of the rate constant for this reaction at (I) 210 oC and (II) 283 oC.
2 lines
(I) By interpolating the graph for when T= 210 oC ( see above) ln k = − 8.8 so k = 1.5 x 10−4 s−1.
(II) By extrapolating the graph for when T= 283 oC (see above) ln k = − 3.7 so k = 2.5 x 10−2 s−1.
 Assignment:
Assignment: ![]() Questions on Topic 16.2: Activation energy
Questions on Topic 16.2: Activation energy


 IB Docs (2) Team
IB Docs (2) Team 