2 &12. Atomic structure (2)
 Atomic structure (2)
Atomic structure (2)
For each question choose the answer you consider to be the best.
1. What is the symbol for a species that contains 8 protons, 9 neutrons and 10 electrons?
A. 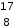 N3−
N3−
B. 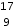 F−
F−
C. 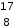 F−
F−
D. 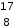 O2−
O2−
2. How many protons, neutrons and electrons are present in an atom of 40Ar?

3. Which species represents a pair of isotopes?

A. W and X
B. X and Y
C. X and Z
D. W and Z
4. What is the difference between the two neutral atoms 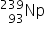 and
and 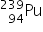 ?
?
A. The number of protons, neutrons and electrons
B. The number of protons and electrons only
C. The number of protons and neutrons only
D. The number of neutrons only
5. The relative atomic mass of bromine is 79.9. It consists of two isotopes 79Br and 81Br.
What is the percentage of 81Br in a sample of naturally occurring bromine?
A. 40%
B. 45%
C. 50%
D. 55%
6. The first successive eight ionization energies for an element are 1260, 2300, 3850, 5150, 6540, 9330, 11000 and 33600 kJ mol-1 respectively. Which group of the periodic table does the element belong to?
A. 3
B. 8
C. 17
D. 18
7. An element shows three peaks in its mass spectrum below. What is the relative atomic mass of the element?

A. 23.0
B. 24.6
C. 25.0
D. 25.7
8. Which electronic transition energy enables the ionization energy of hydrogen to be calculated?
A. n = 1 to n = 2
B. n = 1 to n = ∞
C. n = 2 to n = ∞
D. n = 2 to n = 1
9. Which ion will be deflected the most in a mass spectrometer?
A. 12C+
B. 13C+
C. 12C2+
D. 13C2+
10. Which is correct about the infrared and ultraviolet regions of the spectrum?

11. Which process produces the first line in the visible region of the electromagnetic spectrum for the emission spectrum of hydrogen?
A. The release of energy by electrons
B. The absorption of energy by electrons
C. The deflection of energy by electrons
D. The convergence of energy by electrons
12. What is the electron configuration of the Cr3+ ion?
A. [Ar]4s13d5
B. [Ar]4s23d1
C. [Ar]3d3
D. [Ar]4s13d2
13. What is the electron configuration of an atom with Z = 29?
A. 1s22s22p63s23p64s13d10
B. 1s22s22p63s23p63d9
C. 1s22s22p63s23p64s23d9
D. 1s22s22p63s23p64p23d9
14. In what order do the orbitals increase in energy within the same level?
A. d < f < p < s
B. f < d < p < s
C. s < d < p < f
D. s < p < d < f
15. Which atoms have one or more unpaired electrons in their ground state?
I. Copper
II. Chromium
III. Zinc
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
16. How many unpaired electrons are there in the Ni2+ ion?
A. 0
B. 2
C. 4
D. 6
17. How many d electrons in total are present in an atom of iodine?
A. 10
B. 20
C. 24
D. 48
18. How many orbitals are there in the n = 4 level of an atom?
A. 4
B. 9
C. 16
D. 32
19. Which are correct about energy levels and electron orbitals?
I. The number of d orbitals in the 2d energy level is five.
II. The maximum number of electrons in the third energy level is 18.
III. The two electrons in each full s orbital have opposite spin.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
20. Which equation represents the second ionization energy of a metal M?
A. M(g) → M2+(g) + 2e−
B. M2+(g) → M3+(g) + e−
C. M(s) → M2+(g) + 2e−
D. M+(g) → M2+(g) + e−
 Answers
Answers
1. D, 2. A, 3. D , 4. A, 5. B, 6. C, 7. B, 8. B, 9. C, 10. B,
11. A, 12. C, 13. A, 14. D, 15. A, 16. B., 17. B, 18. C, 19. C, 20. D.
Download the Atomic structure ![]() Topics 2 & 12 MC (2) test
Topics 2 & 12 MC (2) test ![]()
Download the answer grid with the correct answers to cut out for Topics 2 & 12 MC (2) ![]()

 IB Docs (2) Team
IB Docs (2) Team