MC test: Electrons in atoms
 Multiple choice test on 12.1 Electrons in atoms
Multiple choice test on 12.1 Electrons in atoms
Use the following 'quiz' to test your knowledge and understanding of this sub-topic. You will need access to a periodic table (Section 6 of the IB data booklet).
If you get an answer wrong, read through the explanation carefully to learn from your mistakes.
Which equation describes the second ionization energy of copper?
All the species must be in the gaseous state. The second ionization energy involves removing one electron from Cu+(g) ions.
Which transition between energy levels involves the most endothermic energy change?
Energy needs to be put in (i.e. an endothermic process) to promote an electron from a lower to a higher level. The greatest difference between energy levels is between levels 1 and 2.
The wavelength of light for the convergence line in the emission hydrogen spectrum corresponding to the transition from n= 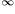 to n=1 is equal to 9.12 x 10-8 m. Which expression is equal to the ionization energy of hydrogen in J mol-1? (h = Planck's constant, L = Avogadro's constant and c = velocity of light).
to n=1 is equal to 9.12 x 10-8 m. Which expression is equal to the ionization energy of hydrogen in J mol-1? (h = Planck's constant, L = Avogadro's constant and c = velocity of light).
Use c = λν and E = hν to give E = h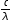 for one electron then substitute the actual value for the wavelength and multiply by Avogadro's constant for 1 mol of electrons. Alternatively look at the units. Units of E are J mol-1. Since the units of h are J s, the units of c are m s-1, the units of λ are m and the units of Avogadro's constant are mol-1 then E must equal (h x c x L) ÷ λ.
for one electron then substitute the actual value for the wavelength and multiply by Avogadro's constant for 1 mol of electrons. Alternatively look at the units. Units of E are J mol-1. Since the units of h are J s, the units of c are m s-1, the units of λ are m and the units of Avogadro's constant are mol-1 then E must equal (h x c x L) ÷ λ.
The graph shows a plot of the 1st ionization energies of the first twenty elements against atomic number.

Which statements about the graph are correct?
I. The linear shapes between B and N and between Al and P provide evidence for Hund's rule.
II. The differences between the values for He and Li and between Ne and Na provide evidence for main energy levels.
III. The drop in values between B and Be and between Al and Mg are due to p sub-levels being lower in energy than s sub-levels.
The straight lines between B & N and between Al & P are due to the three p orbitals filling singly i.e. Hund's rule. The drops show that He and Ne have full main levels and a new outer levels start with Li and Na. B is lower than Be and Al is lower than Mg because the p sub-level is higher in energy than the s sub-level so less energy is required to remove an unpaired electron from B or Al than it takes to remove one of the spin-paired electrons from an s sub-level in Be or Mg.
The first eight ionization energies of an element are 707, 1410, 2940, 3930, 7780, 9900, 12200 and 14600 kJ mol-1 respectively.
Which group in the periodic table does the element belong to?
The big 'jump' in ionization energies occurs after four electrons have been removed so it will be in group 14 (the element is actually tin).
Which statement best explains why the 1st ionization energy of oxygen is lower than the 1st ionization energy of nitrogen?
Oxygen is 1s22s22px22py12pz1 and N is 1s22s22px12py12pz1. The spin-paired electrons in oxygen repel each other so it is easier to remove one of these than one of the three p electrons in N which all have the same spin.
What are the units of Planck's constant?
E = hν so h = E ÷ ν. The units of E are J and the units of ν (frequency) are s-1 so the units of h must be J s.
Which transition will produce a line in the infrared region in the emission spectrum of hydrogen?
Electrons falling to the n = 1 level produce lines in the ultraviolet region of the spectrum, to the n = 2 level in the visible region and to n = 3 (or 4 or 5 ...) in the infrared region as the levels progressively get closer so less energy is emitted during the transitions.
Which electronic configuration gives rise to the largest increase between the second and third ionization energies?
Once two electrons have been removed from 1s22s2 the third electron needs to be removed from the 1s level which is closest to the nucleus and therefore requires the most energy. (The actual values for the 2nd and 3rd ionization energies of beryllium are 1760 and 14800 kJ mol-1 respectively).
Which transition will have the highest energy?
Na+ and Mg2+ will be the two highest as the electron is being removed from a main energy level closer to the nucleus. Mg2+ will be higher than Na even though both of them have the electron configuration of Ne, 1s22s22p6, as Mg2+ has a greater nuclear charge than Na+. (The actual values for F(g), Mg2+(g), Na+(g) and Al2+(g) are 1681, 7740, 4560 and 2740 kJ mol-1 respectively.)

 IB Docs (2) Team
IB Docs (2) Team