Ionic bonding & structure

 4.1 Ionic bonding & structure (2 hours)
4.1 Ionic bonding & structure (2 hours)
Pause for thought
 Something that always amazes me is how, having literally just covered Topic 3 on Periodicity, teachers and books seem to forget all that they have taught on that topic when they introduce ionic bonding. Almost all books and I suspect many teachers make the most elementary mistakes which both they and their students should recognise from the material covered under periodicity are completely wrong.
Something that always amazes me is how, having literally just covered Topic 3 on Periodicity, teachers and books seem to forget all that they have taught on that topic when they introduce ionic bonding. Almost all books and I suspect many teachers make the most elementary mistakes which both they and their students should recognise from the material covered under periodicity are completely wrong.
These mistakes include:
![]() Showing how a sodium atom gives an electron to a chlorine atom when the reaction is actually between sodium metal and gaseous chlorine molecules, Cl2 (see the image on the right showing sodium metal burning in chlorine gas).
Showing how a sodium atom gives an electron to a chlorine atom when the reaction is actually between sodium metal and gaseous chlorine molecules, Cl2 (see the image on the right showing sodium metal burning in chlorine gas).
Na(s) + ½Cl2(g) → NaCl(s)
![]() Making statements such as sodium atoms "want to lose an electron to gain a noble gas configuration (or arrangement)". Apart from the fact that sodium atoms do not have wants, much more important is that they do not easily lose an electron. The process is endothermic to the tune of + 496 kJ mol-1.
Making statements such as sodium atoms "want to lose an electron to gain a noble gas configuration (or arrangement)". Apart from the fact that sodium atoms do not have wants, much more important is that they do not easily lose an electron. The process is endothermic to the tune of + 496 kJ mol-1.
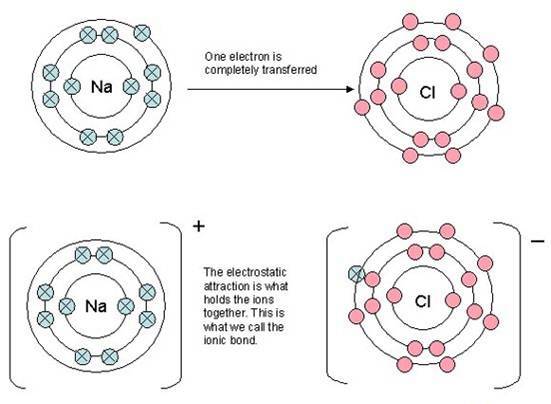
A typical way of showing ionic bonding that can be found in many books
![]() Showing atoms of sodium and chlorine to both be the same size, i.e. with identical atomic radii.
Showing atoms of sodium and chlorine to both be the same size, i.e. with identical atomic radii.
![]() Showing a chloride ion the same size as a chlorine atom, and in some cases the same size as a sodium ion.
Showing a chloride ion the same size as a chlorine atom, and in some cases the same size as a sodium ion.
![]() Showing all the energy levels in both the sodium and chlorine atoms to be equally spaced.
Showing all the energy levels in both the sodium and chlorine atoms to be equally spaced.
![]() Showing the electrons separately in the first energy level and in pairs in the subsequent energy levels.
Showing the electrons separately in the first energy level and in pairs in the subsequent energy levels.
![]() Ignoring the changes of state that take place during the reaction.
Ignoring the changes of state that take place during the reaction.
So before you teach ionic bonding – just reflect a while!
Nature of Science
Natural phenomena can be explained by using theories. For example, molten ionic compounds conduct electricity but solid ionic compounds do not - this can be explained in terms of the breaking of ionic lattices.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesStudents should be familiar with formulas and the names of the following polyatomic ions: NH4+, OH–, NO3–, HCO3–, CO32–, SO42– and PO43–. International-mindedness(Nothing is listed in the programme for international-mindedness under this sub-topic) |
Teaching tipsIt is usual to use sodium chloride as the classic example. For both Standard level and Higher Level students I start off by asking them all the questions to get them to revise what they learned in Topic 3 to avoid the sort of mistakes listed above. It can be good to get them to then look critically at what is written in many text books about ionic bonding. For Higher Level students I do teach this slightly differently. I get them to think about all the processes involved e.g. changing the sodium solid into sodium gas before they can use the ionization energy and breaking the Cl-Cl covalent bond before introducing the idea of electron affinity. I then get them to look at all the energy terms to form just the ions and realise that apart from the electron affinity they are all positive (and that is positive for forming an oxide salt too). This then leads to the idea that what drives the reaction is the extremely exothermic formation of the ionic lattice and this explains what exactly an ionic bond is. What this does is introduce them to the idea of a Born-Haber cycle without actually covering it properly (I leave that to Topic 15). I do a simplified 'watered down' version for Standard Level students as they do not need the Born- Haber idea (even though they do cover Hess's law and energy cycles in Topic 5) but it is still good for them to see what the driving process is for the reaction. Stress that an ionic bond is not directional and it is not the attraction between one sodium ion and one chloride ion. After covering sodium chloride I get them to work out how ionic salts such as magnesium chloride, calcium oxide and aluminium oxide could be formed by similar processes. This then leads on to the formulas of ionic compounds involving polyatomic ions such as sodium nitrate etc. | Study guide
Page 25 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Ionic bonding & structure. For short-answer questions which can be set as an assignment for a test, homework or given for self study together with model answers see Ionic bonding & structure questions. For a data response question on ionic bonding see Data response question with the worked answers on a separate page Data response answers. Vocabulary listelectrovalent IM, TOK, & 'Utilization' etc.See separate page which covers all of Topic 4. Practical work |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A very simple UK A Level revision video which shows the electron transfers taking place between neutral atoms to form ions. It leads on to a second video which discusses giant ionic lattices and how these explain the properties of ionic compounds. Remember that "dot and cross" diagrams are the UK way of describing Lewis structures!
2. A video by the Khan academy on bonding which is supposed to be educational. To me this exemplifies all that is wrong about how ionic bonding is sometimes taught. No questions are asked of students and the facts given are often simply wrong.
![]() Iconic (!) bonding (Khan academy)
Iconic (!) bonding (Khan academy)

 IB Docs (2) Team
IB Docs (2) Team 















