Graphical techniques

 11.2 Graphical techniques (2 hours)
11.2 Graphical techniques (2 hours)
Pause for thought
During the past decades the IB has used the same graph several times as the basis of a question. The latest is in the specimen papers for the new programme. The graph used is:
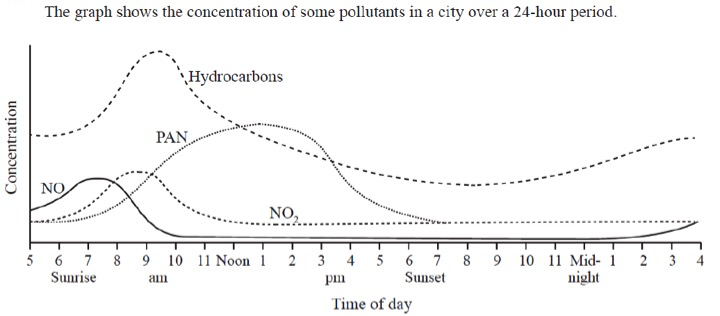
It is an interesting graph because it shows how primary pollutants build up during the morning rush hour periods and then the secondary pollutants, PAN (peroxyacylnitrates), increase during sunlight hours as the nitrogen oxides undergo photochemical reactions with the hydrocarbons. Because this is very much the focus of the questions asked, attention is drawn away from the fact that if a student drew such a graph when they were expressing their data graphically they would probably be penalized. It might be worth showing this graph to students and ask them to comment critically on it.
Firstly, is it a 'drawn' graph or a 'sketch' graph? It does not seem to fit either of the IB definitions so would seem to lie somewhere between the two. One of the axes does have a scaled axis (drawn graph) but the other does not have a scaled axis (sketch graph). Other problems include the fact that no units are given for the concentration - is the same unit of concentration being used for all four pollutants? No units are actually given for the time of day either although you are presumably meant to deduce that these are in hours. The title says' 'for a 24 hour period' but the exact date is not given - neither is the location of the city/town where the measurements were made. It could be that this was for an atypical day at an unusual location etc. etc. It might also be worth considering why NO and hydrocarbons build up during the morning rush hour but not during the evening rush hour - maybe nobody travelled back home that day?.
Graphing is a powerful tool but it is also a tool that can be used to manipulate interpretation. You only have to look in the financial press to see how graphs are often misused. For example, the press reported that Facebook shares plummetted after an announcement by the Chief Financial Officer, David Wehner in October 2014. A superficial look at the graph might lead you to conclude that the value of the company had almost been wiped out but on closer inspection of the y axis it can be seen that the company only lost about 9% of its value - hardly a 'plummet'.

Nature of Science
Graphs can be used to find connections between different variables - the idea of correlation.
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesNo clarification notes are given in the Guide for this sub-topic. International-mindednessBecause they are visual, charts and graphs mainly transcend language barriers so can facilitate communication between scientists worldwide. |
Teaching tipsYou will need to decide whether to teach this topic as a separate sub-topic or whether to simply include its contents as you cover graphing techniques elsewhere. Personally I think it is better to incorporate it as and when it arises. For example, sketched graphs can be covered under the gas laws (e.g. the relationship between P and V for a fixed mass of gas at a constant temperature) and under rates of reaction showing how the change in concentration depends upon time for first and second order reactions etc. For quantitative coverage, experiments such as Enthalpy changes and Determining Ea for a reaction can be used to show extrapolation (to determine the Arrhenius constant, A), Analysis of Cu(II) ions in solution using a spectrometer can be used to show interpolation and Reaction rates and Determining Ea for a reaction (for determining the activation energy, Ea) for taking tangents. You will need to ensure that students can both sketch and draw their own graphs. Graphs should have a title, properly labelled axes with the appropriate units and a suitable scale such that all or most of the space is utilised correctly. Students should also know that a minimum of five data points is required before a line or curve of best-fit can have real validity. Error bars and detailed statistical analysis of graphs, e.g. t-tests are not required for IB Chemistry. | Study guide
Page 100 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Graphical techniques. For short-answer questions see Graphical techniques questions together with the worked answers on a separate page Graphical techniques answers. Vocabulary listsketch IM, TOK, Utilization etc.See separate page which covers all of Topic 11 Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. A video on interpreting scientific graphs by Jaime Jackson
Interpreting data - Analyzing graphs ![]()

 IB Docs (2) Team
IB Docs (2) Team 

















