Halogenoalkanes & benzene

 10.2 Functional group chemistry (6.5 hours)
10.2 Functional group chemistry (6.5 hours)
3. Halogenoalkanes & benzene (estimated 1.5 hours)
Pause for thought
The syllabus states:
“Halogenoalkanes are more reactive than alkanes.”
A rather nice Nature of Science exercise might be to get your students to question the validity of this statement. Is it a valid statement that is always true?
It is certainly true that alkanes undergo very few different types of chemical reactions. This is mainly due to the relative strengths of the C−H and C−C bonds and the fact that carbon cannot expand its octet. Contrast this with many halogenoalkanes where the carbon atom bonded to the halogen atom is electropositive and can undergo substitution reactions with nucleophiles such as the hydroxide ion, the cyanide ion or ammonia. The statement made in the IB Guide clearly does have some validity. However it is certainly not always the case. One of the hazards associated with aircraft and other vehicles that use alkanes as a fuel is that if there is a fuel leak due to an accident then there is a very real danger of fire. All alkanes are very reactive when it comes to burning them in oxygen. Compare this with halogenoalkanes, in particular fluorocarbons. Fluorine is a highly electronegative element which will make the carbon atom attached to it very electropositive so fluorocarbons would be expected to react readily with nucleophiles. In fact they are completely inert. This is due to the very strong C−F bond. The irony is that certain halogenoalkanes (e.g. bromochlorodifluoromethane, known as halon 1211) are so unreactive that they are carried in aircraft to combat alkane fires – in this instance alkanes are very definitely much more reactive than halogenoalkanes.
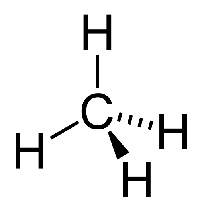
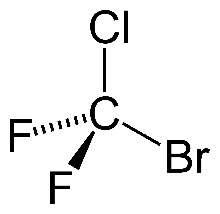 When it comes to combustion, halon1211 (left), a halogenoalkane, is much less reactive than methane (right), an alkane.
When it comes to combustion, halon1211 (left), a halogenoalkane, is much less reactive than methane (right), an alkane.
Another good example to show that in many cases halogenoalkanes are actually less reactive than alkanes is to compare the polymers poly(tetrafluoroethene), PTFE, and poly(ethene). PTFE, which is also known as Teflon®, is the 'non-stick' polymer which is so inert that it is used to coat cooking utensils. According to Plastech the only chemicals known to affect Teflon® finishes are certain alkali metals and some of the most highly reactive fluorinating agents such as xenon difluoride and cobalt(III) fluoride. Compared to this poly(ethene) is much more reactive.
Nature of Science
Organic chemical reactions involving functional group interconversions are among the key factors responsible for the progress made in the development and applications of scientific research.
Learning outcomesAfter studying this sub-topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesSN1 and SN2 mechanisms of nucleophilic substitution are not required. International-mindednessIt is perhaps worth mentioning that benzene is one chemical that is banned for use in school laboratories in almost every country in the world. |
Teaching tipsI usually start off by asking students why the topic is limited to chloro-, bromo, and iodoalkanes but ignores fluoroalkanes. Hopefully this will re-enforce the importance of bond enthalpies as the C-F bond enthalpy (492 kJ mol-1) is so much stronger than the C-Cl bond enthalpy (324 kJ mol-1). (In fact carbon-halogen bond enthalpies are also important in Topic 20 explaining why iodoalkanes are more reactive towards nucleophilic substitution than chloroalkanes). It is quite difficult to know how far to go with this topic at Standard Level. Students are supposed to understand the concept of a nucleophile and why nucleophiles are attracted to the δ+ carbon atom of the halogenoalkane to bring about a substitution reaction. It is reasonable to expect them to understand that water, cyanide ions, hydroxide ions and ammonia can all act as nucleophiles as they all have at least one non-bonding pair of electrons. They could then probably deduce the organic products from these reactions but the syllabus clearly states that they only need to write equations for the reaction of halogenoalkanes with aqueous sodium hydroxide to form alcohols. There is no need to talk about the two different mechanisms of nucleophilic substitution or presumably use curly arrows. Similarly the amount of chemistry they need to know about benzene at this level is extremely limited. It makes sense to cover everything about benzene in one go. I would recommend combining the physical and chemical evidence for the structure of benzene, which is supposed to be covered in sub-topic 10.1, with the fact that it cannot readily undergo addition reactions due to the extra stability of the benzene ring. This is caused by the resonance structures (delocalization) and is equal to 150 kJ mol-1 as shown by comparing the enthalpy of hydrogenation of benzene with three times the enthalpy of hydrogenation of cyclohexene. It is worth doing the attached practical as it really does show that the speed at which the various classes of halogenoalkanes undergo nucleophilic substitution can vary enormously and it reinforces the idea of primary, secondary and tertiary halogenoalkanes. However the explanation for much of it is really Higher Level. | Study guide
Page 88 QuestionsFor ten 'quiz' multiple choice questions with the answers explained see MC test: Halogenoalkanes & benzene. For short-answer questions on halogenoalkanes & benzene which can be set as an assignment for a test, homework or given for self study together with model answers see Halogenoalkanes & benzene questions. Vocabulary list:nucleophile IM, TOK, Utilization etc.See separate page which covers all of Topic 10. Practical work |
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. One of the problems with videos about nucleophilic substitution is that many of them also go into the SN1 and SN2 mechanisms which are not on the core part of the syllabus. This one, by Master Organic Chemistry, just covers the basics of what a nucleophilic substitution reaction is.
2. There is a similar problem with videos about the structure of benzene at this level as most of them go into the delocalization of pi electrons which is not on the core part of the programme. This one by Allery tutors does cover some of the physical evidence that shows that benzene does not simply consist of three alternate C=C double bonds in a six-membered ring.

 IB Docs (2) Team
IB Docs (2) Team 











