Identification from spectra - Question 17
 Spectroscopic identification of organic compounds: Question 17
Spectroscopic identification of organic compounds: Question 17
This page on identifying a compound from its spectra can be marked as direct student access either for assigning it as a test or for students to work on in their own time. If you do not wish to use student access, links to downloadable versions of the question and, separately the worked answer, can be found at Printable versions of written tasks.
Identify Compound Q and explain how the data from each of (a) to (d) provides the information to support your answer.
(a) Elemental analysis of Compound Q

(b) Mass spectrum of Compound Q

(c) Infrared spectrum of Compound Q
(d) 1H NMR data for Compound Q
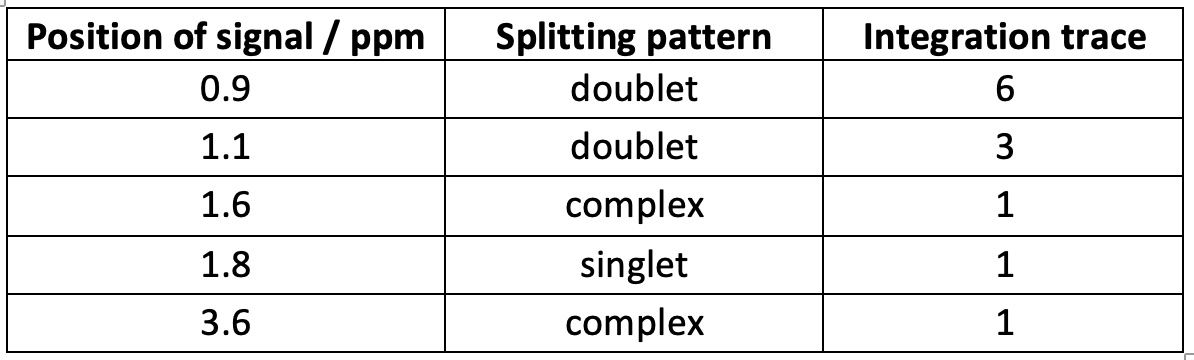
1H NMR data from https://spectrabase.com/spectrum/LnpPkfzUaE2

 IB Docs (2) Team
IB Docs (2) Team 
.png)