Question 1
Which of the following chemical equations correctly represents one method for how carbon is made available for absorption by aquatic autotrophs?
Which of the following chemical equations correctly represents one method for how carbon is made available for absorption by aquatic autotrophs?
Some students were investigating the production of carbon dioxide from anaerobic respiration in yeast at different temperatures. They had access to the apparatus to carry out two different methods.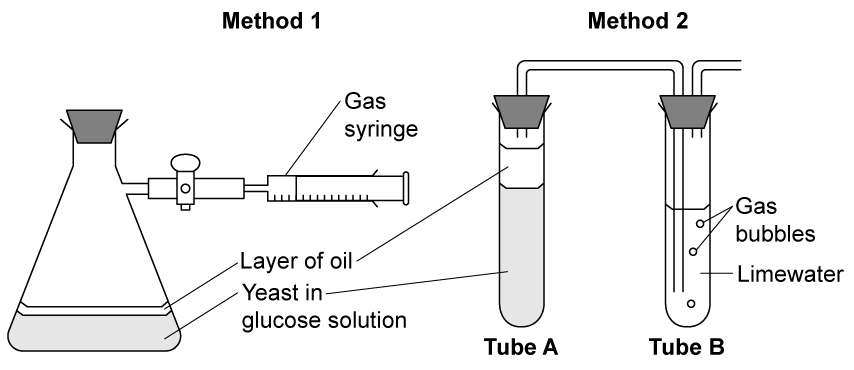
Method 1: Using a gas syringe, students collected gas produced by yeast at different temperatures.
Method 2: Using limewater and a delivery tube, students counted the number of bubbles of gas produced by the yeast at different temperatures, and used limewater to show that it was carbon dioxide.
Which method should the students use and why?
Method 1 should be used because a statistical analysis can be carried out on method 1 but not method 2
Method 2 proves that the gas produced is carbon dioxide, this gives more valid results
Method 1 should be used because it provides quantitative measurements, which will be more accurate
Method 2 should be used because it gives qualitative and quantitative results so is more accurate
A company is advertising a peat-free compost with the statement below:
'Our peat-free compost is perfect for supporting growth in your plants whilst protecting the environment'
Which statement best justifies the use of this statement in the advertising of this peat-free compost?
Compost with peat in it may contain partially digested organisms
Peat-free compost will contain a lower proportion of fungi which may parasitise crop plants
Using peat-free compost reduces demand for the peat bogs which are an unsustainable resource
Peat-free compost contains more nutrients for maximum plant growth
The pH content of a water body in Australia was measured over a 10 year period. Scientists are concerned about the general downward trend of the data, which shows less alkali conditions than is characteristic.
Which of the statements suggests why the scientists may have cause for concern?
I and II only
II and III only
None of the above
II only
The graph below shows the daily fluctuations in pH in a freshwater pond over a 3 day period.
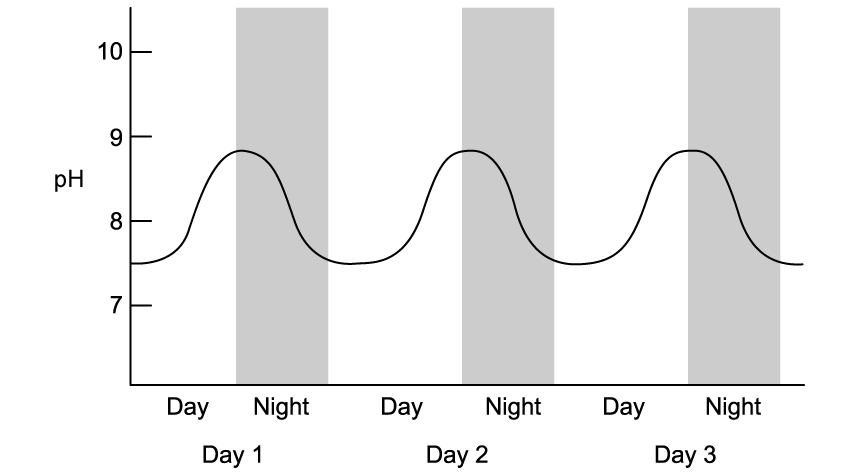
Which statement correctly explains the fluctuations shown in the data?
pH decreases as a result of CO32- ions which are formed when carbon dioxide combines with water molecules
pH decreases when the rate of photosynthesis increases and CO2 is removed from the water by aquatic plants
pH decreases due to the presence of more H+ ions produced in the dissociation of carbonic acid
pH decreases during the day when more dissolved CO2 enters the water