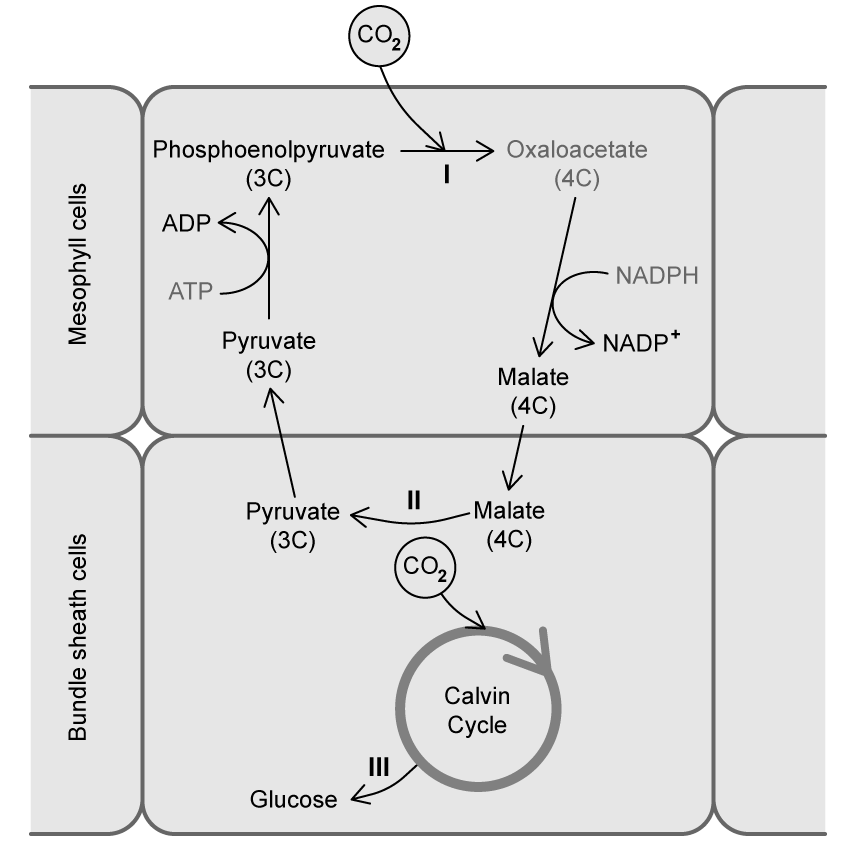
a)
Suggest, with a reason, which label (I , II , or III ) indicates a catabolic reaction.
[2 marks]
Assess your score
View Answer
b)
The diagram in part (a) illustrates how life is based on certain biochemical compounds.
Identify which major group of carbon-containing compounds is dominant in this diagram.
[1 mark]
Assess your score
View Answer
c)
Fats and cholesterol are essential to structures and functions in the bodies of animals and therefore need to be transported in blood.
Discuss how these molecules are transported.
[ 3 marks ]
Assess your score
View Answer
d)
Draw a labelled diagram of a generalised amino acid.
[4 marks]
Assess your score
View Answer
Next Question
a)
Eastern collared lizards and pigs are two examples of animals that pant to keep cool.
Suggest how panting helps these animals to cool down.
[ 2 marks ]
Assess your score
View Answer
b)
Methane is a product of the incomplete breakdown of dead organic matter. Methane can be seen bubbling out of the water in volcanic springs.
Explain why, once methane has been produced, it bubbles off the water.
[2 marks]
Assess your score
View Answer
c)
Draw a labelled diagram showing cohesive forces between three water molecules.
[3 marks]
Assess your score
View Answer
Previous Question Next Question
a)
Outline the significance of the surface tension of water to living organisms.
[2 marks]
Assess your score
View Answer
b)
Using your knowledge of the properties of water, explain why dilute aqueous solutions (eg. glucose solution) are clear.
[2 marks]
Assess your score
View Answer
c)
Explain the relationship between the osmolarity of a solution and its water potential.
[3 marks]
Assess your score
View Answer
d)
Explain how water's high specific heat capacity helps to keep environmental conditions constant for organisms.
[2 marks]
Assess your score
View Answer
Previous Question Next Question
a)
Water exists in all three of its physical states in Nature.
Give an example of each physical state of water that exists in Nature and how it affects organisms and ecosystems.
[6 marks]
Assess your score
View Answer
b)
Compare and contrast the properties of methane and water.
[4 marks]
Assess your score
View Answer
c)
Explain why a methane molecule (CH4 ) forms a tetrahedral shape.
[2 marks]
Assess your score
View Answer
Previous Question Next Question
One mark is available for clarity of communication throughout this question.
a)
Multiple pieces of evidence are required for scientists to falsify theories.
Discuss the evidence that scientists used to falsify the theory of vitalism.
[3 marks]
Assess your score
View Answer
b)
Deamination (the removal of an amino group from a molecule) and gluconeogenesis (the production of glucose from non-carbohydrate sources) are two reactions that occur in the liver.
From the information given, suggest with reasons, which metabolic reactions these are classified as.
[4 marks]
Assess your score
View Answer
c)
Explain the properties of water that are key for the survival of an oak tree.
[8 marks]
Assess your score
View Answer
Previous Question