Question 1a
State the reason why enzymes are referred to as biological catalysts.
[1 mark]
Question 1b
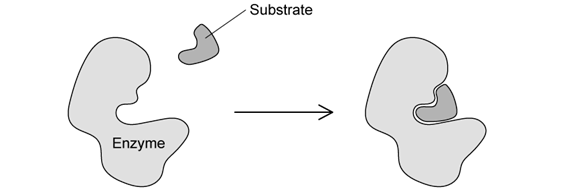
Describe the events taking place in the image.
[3 marks]
State the reason why enzymes are referred to as biological catalysts.
[1 mark]

Describe the events taking place in the image.
[3 marks]
A student wanted to investigate the effect of substrate concentration on the activity of an enzyme called catalase. Catalase is an enzyme that commonly occurs inside living cells where it breaks down toxic hydrogen peroxide into oxygen and water. The image below shows the experimental set up done by the student.
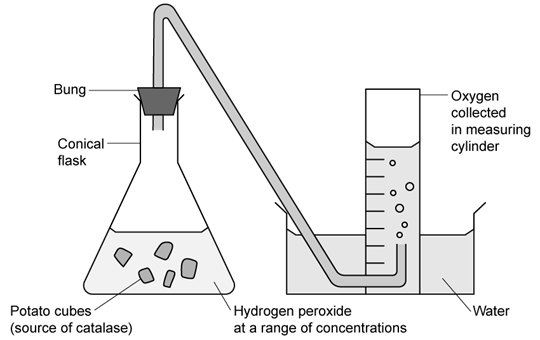
List two control variables that the student would need to be aware of in the experiment shown in the image.
[2 marks]
The student decided to make up solutions at five different hydrogen peroxide concentrations. Their measurements for these solutions are shown in the table below.
| Concentration of hydrogen peroxide solution (%) | Volume of hydrogen peroxide required (cm3) | Volume of distilled water required (cm3) |
| 10 | 10 | 90 |
| 8 | B | C |
| 6 | 6 | 94 |
| A | 4 | 96 |
| 2 | 2 | 98 |
Give the measurements needed to fill in gaps A-C in the table.
[1 mark]
After measuring out the range of hydrogen peroxide concentrations shown in part (b), the student carried out the experiment using the equipment set up in part (a). They recorded the volume of oxygen (the product) produced after one minute, and repeated this measurement three times at each concentration. Their results are shown in the table below.
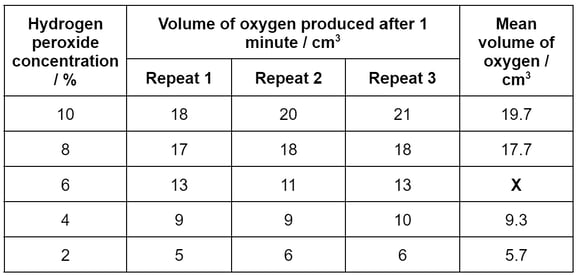
Use the data in the table to calculate the value missing from the square marked X.
[1 mark]
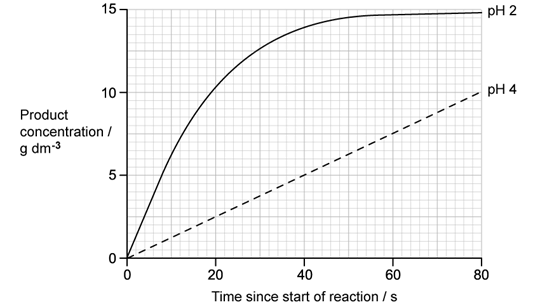
A researcher investigated the effect of pH on the activity of stomach enzyme pepsin.
Their results are shown in the image below.
The rate of reaction can be calculated by using the following formula:
Calculate the rate of reaction at pH 4. Give your answer with the correct units.
[2 marks]
Describe the differences between the curves at pH 2 and pH 4.
[2 marks]
State why product production at pH 2 does not continue indefinitely but reaches a plateau at around 14.75 g.
[1 mark]
d)
Predict the outcome if the pH were increased to pH 10.
[1 mark]
Explain your answer at part i).
[2 marks]
An example of the use of immobilised enzymes in industry is in the production of lactose-free milk.
Identify the enzyme used in this process.
[1 mark]
State the substrate and products of the reaction.
[2 marks]
State the benefits of having a large number of small beads for this process, as opposed to a small number of large beads.
[2 marks]
One mark is available for clarity of communication throughout this question.
List three methods that can be used to immobilise enzymes.
[3 marks]